Limbus corneae stem cell serum-free medium and culture method thereof
A serum-free medium and corneal limbal stem cell technology, applied in the field of stem cell culture, can solve the problems of unsatisfactory stem cell proliferation rate, stem cell purity and quantity, unsatisfactory culture effect, etc., achieve rapid and stable cell source, improve purity and stability , the effect of improving cell quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
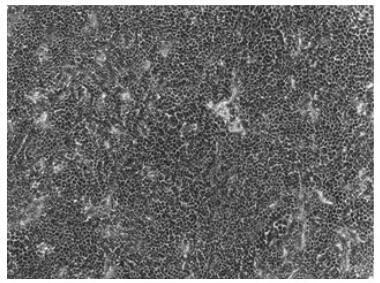
[0065] 1. A serum-free culture medium for limbal stem cells
[0066] A serum-free medium for limbal stem cells, consisting of the following components:
[0067] 216 mL Ham's F12, 216 mL DMEM, 5 mL 100× penicillin-streptomycin double antibody solution, 1 mL human recombinant EGF stock solution, 500 μL insulin stock solution, 500 μL vitamin C stock solution, 500 μL 3,3', 5-triiodo-L-thyronine stock solution, 500 μL hydrocortisone stock solution, 1 μL forskolin stock solution, 0.5 μL manganese sulfate monohydrate, 0.5 μL sodium selenite, 500 μL silicon metasilicate sodium phosphate, 0.5 μL ammonium metavanadate, 0.5 μL nickel chloride hexahydrate, 0.5 μL stannous chloride dihydrate, 0.5 μL ethanolamine, 500 μL phosphoethanolamine, 0.5 μL ammonium molybdate tetrahydrate, 2.7 g 4-hydroxyethyl piperazine ethanesulfonic acid, 10 g bovine serum albumin, 5 mL lipid concentrate and 50 mL serum replacement.
[0068] The concentration of each added component was 10 ng / mL human recombina...
Embodiment 2
[0081] Example 2 Medium formula
[0082] The experimental method is the same as in Example 1, the only difference is that the concentration of each added component in the serum-free medium of limbal stem cells used in this example is:
[0083] 10 ng / mL human recombinant EGF, 10 μg / mL insulin, 5×10 -9 M 3-iodothyronine, 0.2 μg / mL hydrocortisone, 2×10 -5 M Forskolin, 0.5×10 -9 M manganese sulfate monohydrate, 5×10 -7 M sodium selenite, 0.1×10 -3 M sodium metasilicate, 8×10 -6 M ammonium metavanadate, 3×10 -10 M nickel chloride hexahydrate, 8×10 -10 M stannous chloride dihydrate, 3×10 -7 M ethanolamine, 8×10 -6 M Phosphoethanolamine, 1×10 -9 M ammonium molybdate tetrahydrate, 8 g / L 4-hydroxyethylpiperazineethanesulfonic acid, 20 μg / mL vitamin C, 3% bovine serum albumin, 0.5% lipid concentrate, and 15% serum replacement.
Embodiment 3
[0084] Embodiment 3 culture medium formula
[0085] The experimental method is the same as in Example 1, the only difference is that the concentration of each added component in the serum-free medium of limbal stem cells used in this example is:
[0086] 20 ng / mL human recombinant EGF, 5 μg / mL insulin, 1×10 -9 M 3-iodothyronine, 1 μg / mL hydrocortisone, 0.5×10 -5 M Forskolin, 2×10 -9 M manganese sulfate monohydrate, 10×10 -7 M sodium selenite, 1×10 -3 M sodium metasilicate, 3×10 -6 M ammonium metavanadate, 8×10 -10 M Nickel chloride hexahydrate, 3×10 -10 M stannous chloride dihydrate, 8×10 -7 M ethanolamine, 3×10 -6 M Phosphoethanolamine, 6×10 -9 M ammonium molybdate tetrahydrate, 2 g / L 4-hydroxyethylpiperazineethanesulfonic acid, 50 μg / mL vitamin C, 1% bovine serum albumin, 2% lipid concentrate, and 5% serum replacement.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com