Pluripotent embryonic-like stem cells derived from corneal limbus, methods of isolation and uses thereof
a technology of embryonic stem cells and corneal limbs, applied in the field of purified preparations of mammalian pluripotent stem cells, can solve problems such as problems such as problems in stem cell research and treatment, problems associated with the use of es cells, and tissues or cells derived from es cells that are not ideal for medical treatment, and achieve normal karyotype and high telomerase activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0077] 1) Collection of Limbal Tissue Biopsies
[0078] Prior to initiating the collection of limbal tissue biopsies from human patients, Institutional Review Board approval was obtained. Informed consent was obtained from each patient and donor, and all human subjects were treated according to the Helsinki Accord. A 2-3 mm limbal biopsy of the donor eye was collected surgically from superior or temporal quadrants of the corneal surface by lamellar keratectomy. After excision, biopsies were immediately placed in a 2 ml transport vial filled with transport medium. The transport medium consisted of Dulbecco's Modified Eagles Medium (DMEM) and Ham's F-12 Medium (DMEM:F-12; 1:1) supplemented with 5% fetal bovine serum (FBS) or 5% human serum collected from cord blood, 0.5% dimethyl sulphoxide (DMSO), 2 ng / ml recombinant human epidermal growth factor (rhEGF), 5 μg / ml insulin, 5 μg / ml transferrin, 5 μg / ml sodium selenite, 0.5 μg / ml hydrocortisone, 0.1 nmol / l cholera toxin A, 50 μg / ml gentam...
example 2
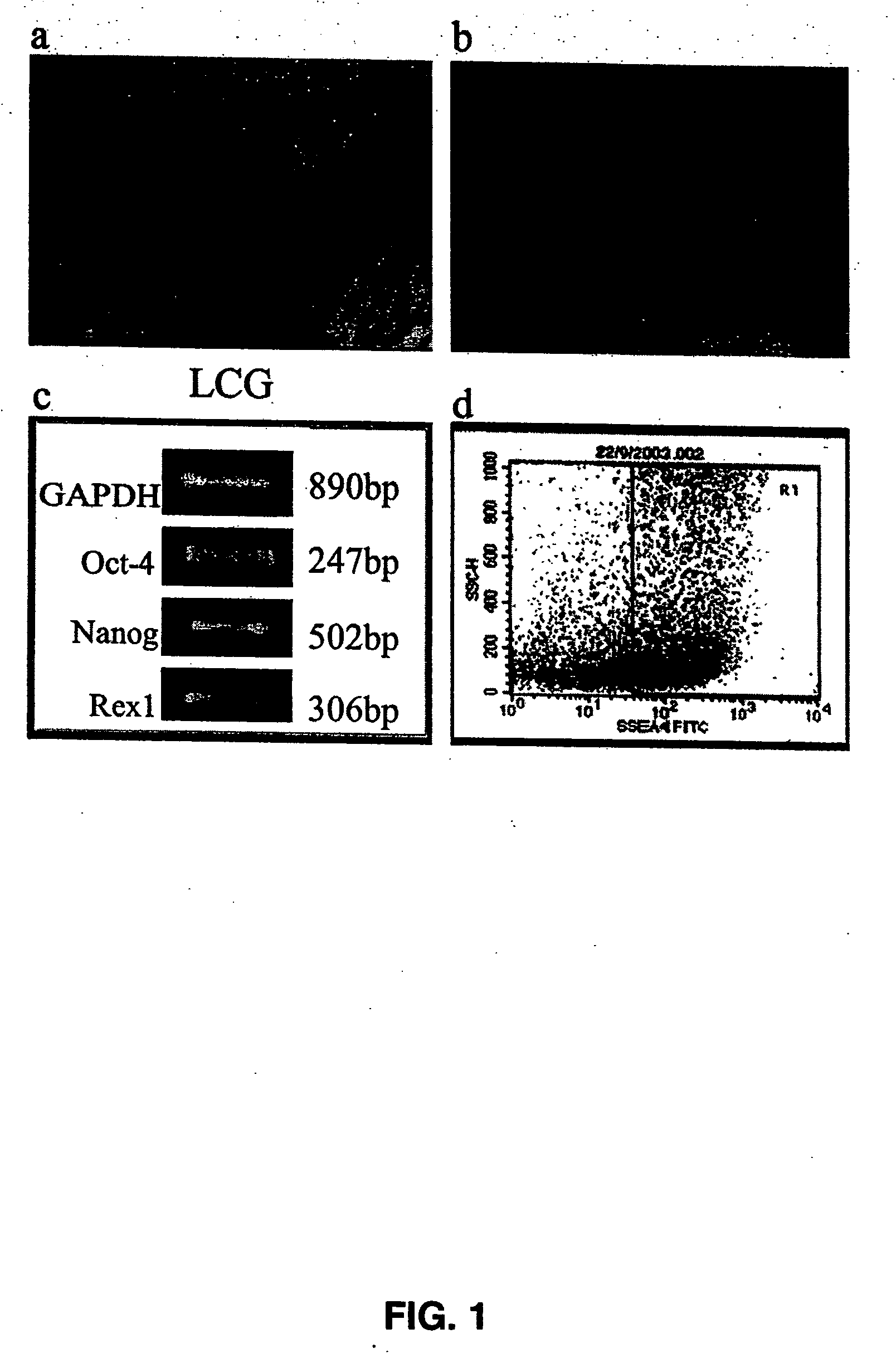
[0094] Analysis and Characterization of Pluripotent Embryonic-Like Stem Cells:
[0095] As outlined in Example 1, pluripotent ELSCs were derived from limbal tissue biopsies. Although not wishing to be limited to any particular theory, it appears that corneal limbus has essentially two stem-cell types that are segregated into two zones. The top layer of the limbus is composed mainly of corneal epithelial stem cells that are P-63 positive, while the basal layer is composed mainly of stromal cells. It appears that the pluripotent ELSCs disclosed herein, predominantly reside in the stromal layer, and may migrate towards the epithelial zone as needed.
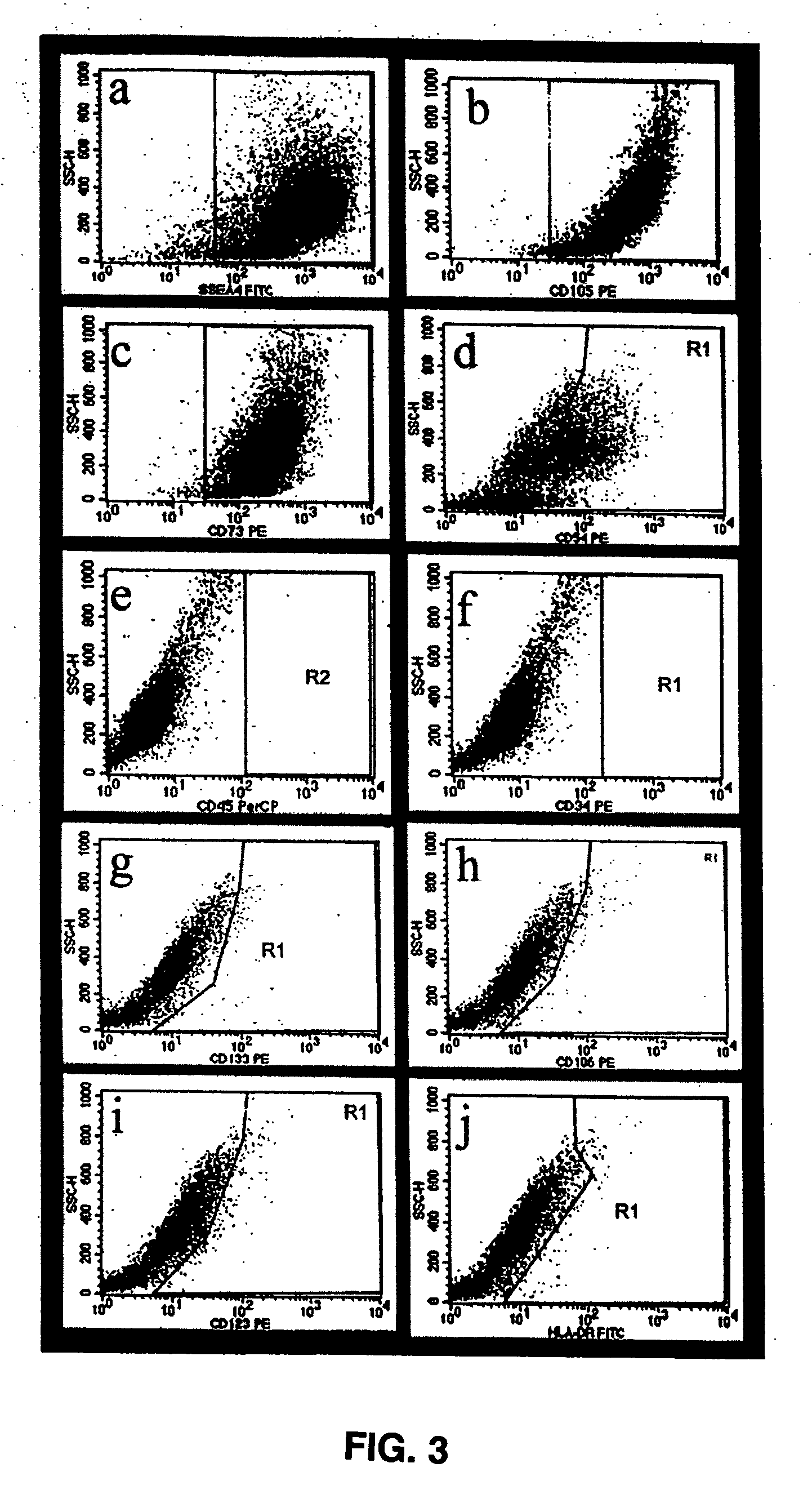
[0096] To better understand the nature of the pluripotent ELSCs derived from limbal tissue, and the undifferentiated status of these cells, ELSCs were analyzed using flow cytometry, immunofluorescence, and molecular analysis for the presence or absence of various cellular markers for undifferentiated and differentiated cells. Karyotype and te...
example 3
[0115] Differentiation and Analysis of Pluripotent Embryonic-Like Stem Cells:
[0116] 1) Generation of Embryoid-Like Bodies from Pluripotent Embryonic-Like Stem Cells
[0117] To determine whether the undifferentiated human pluripotent ELSCs could form embryoid-like bodies (ELBs) in culture, the cells were first allowed to proliferate and the cell cultures expanded. Next, the cells were cultured on bacteriological plates having a non-adhesive surface that prevented attachment of the ELSCs, and stimulated differentiation of these cells. Briefly, ELSCs were dissociated by briefly exposing them to a 0.05% trypsin-EDTA solution, and subsequently cultured as a suspension culture in ES cell medium containing DMEM:F-12 or knockout DMEM, supplemented with 10-20% fetal calf serum, cord blood serum, or knockout serum replacement. The media was also supplemented with β-mercaptoethanol, L-glutamine, insulin, human transferrin, sodium selenite, but did not contain bFGF or hLIF. The cells were incub...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| magnetic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com