Peptide having cell membrane penetrating activity
a cell membrane and peptide technology, applied in the direction of peptide/protein ingredients, antibody medical ingredients, peptide sources, etc., can solve the problems of limited use of in vivo cellular delivery techniques, extremely limited methods for protein delivery, etc., and achieve high efficiency in drug targeting and prominent penetrating efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
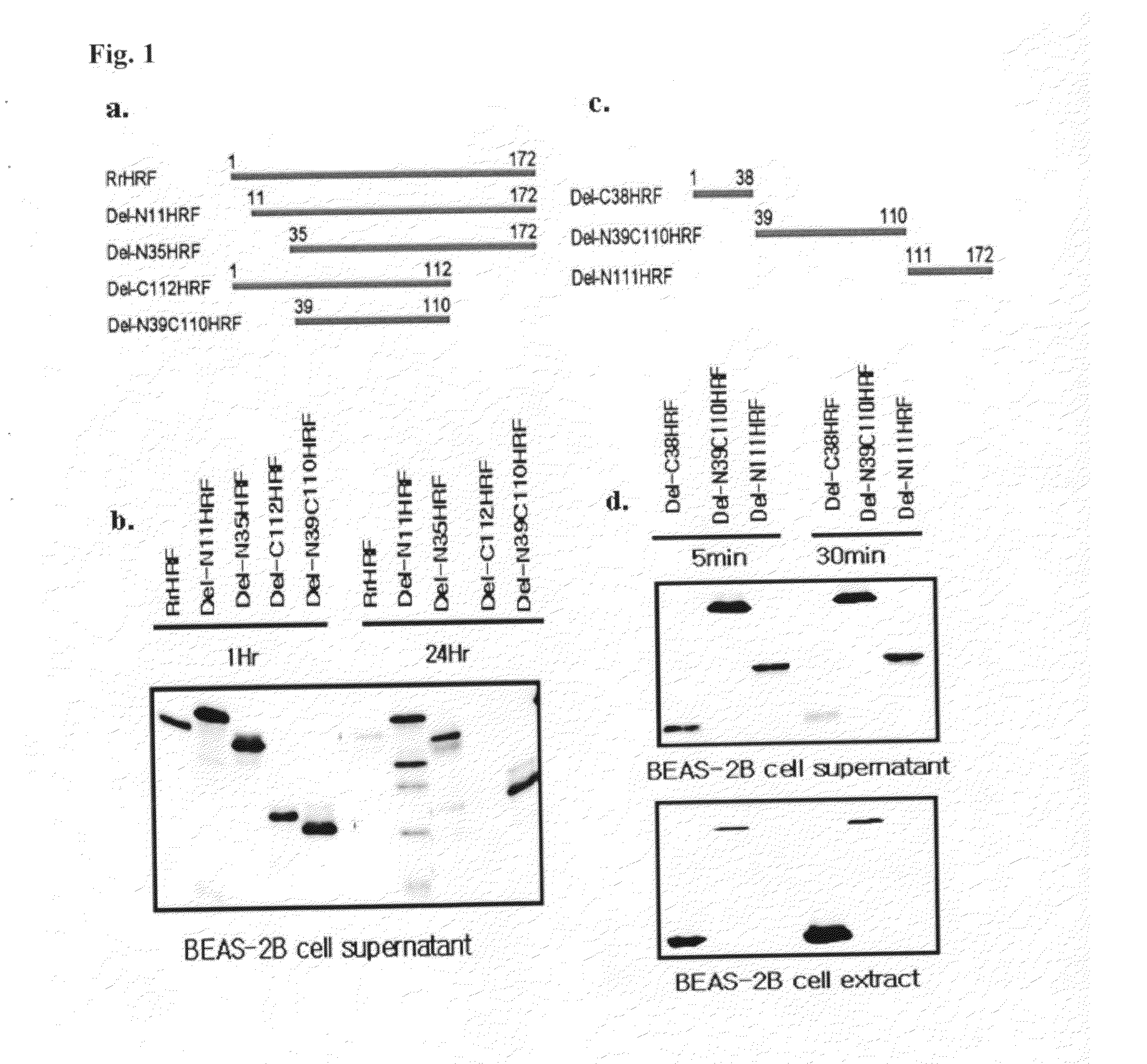
[0103]Mapping of PTD Using Various Deletion Forms of TCTP
[0104]In order to confirm the region of the TCTP acting as PTD, various deletion constructs were prepared and then used in the experiment as follows.
1) Isolation and Purification of Deletion Forms of TCTP
[0105]To overexpress each of those deletion forms of TCTP (FIGS. 1a and 1c), pRSET vector that is capable of tagging 6 histidine was employed. Subcloning with DNA sequences corresponding to each deletion forms of TCTP was performed in the multicloning site of the vector. Then, the recombinant expression vector was introduced into E. coli BL21(DE3)(Novagen) or BL21(DE3)pLysS (Novagen). The expression of the deletion forms of TCTP was induced by IPTG (isopropyl β-D-thiogalactoside) for 3 hours, then the protein was isolated and purified by using Ni column which binds to polyhistidine.
2) Cell Culture and Treatment with the Protein
[0106]BEAS-2B cell was treated with the deletion form of TCTP at the concentration of 15 ug / ml for 1 ...
example 2
Confirmation of Cell Penetrating Efficiency of the Peptide of the Present Invention
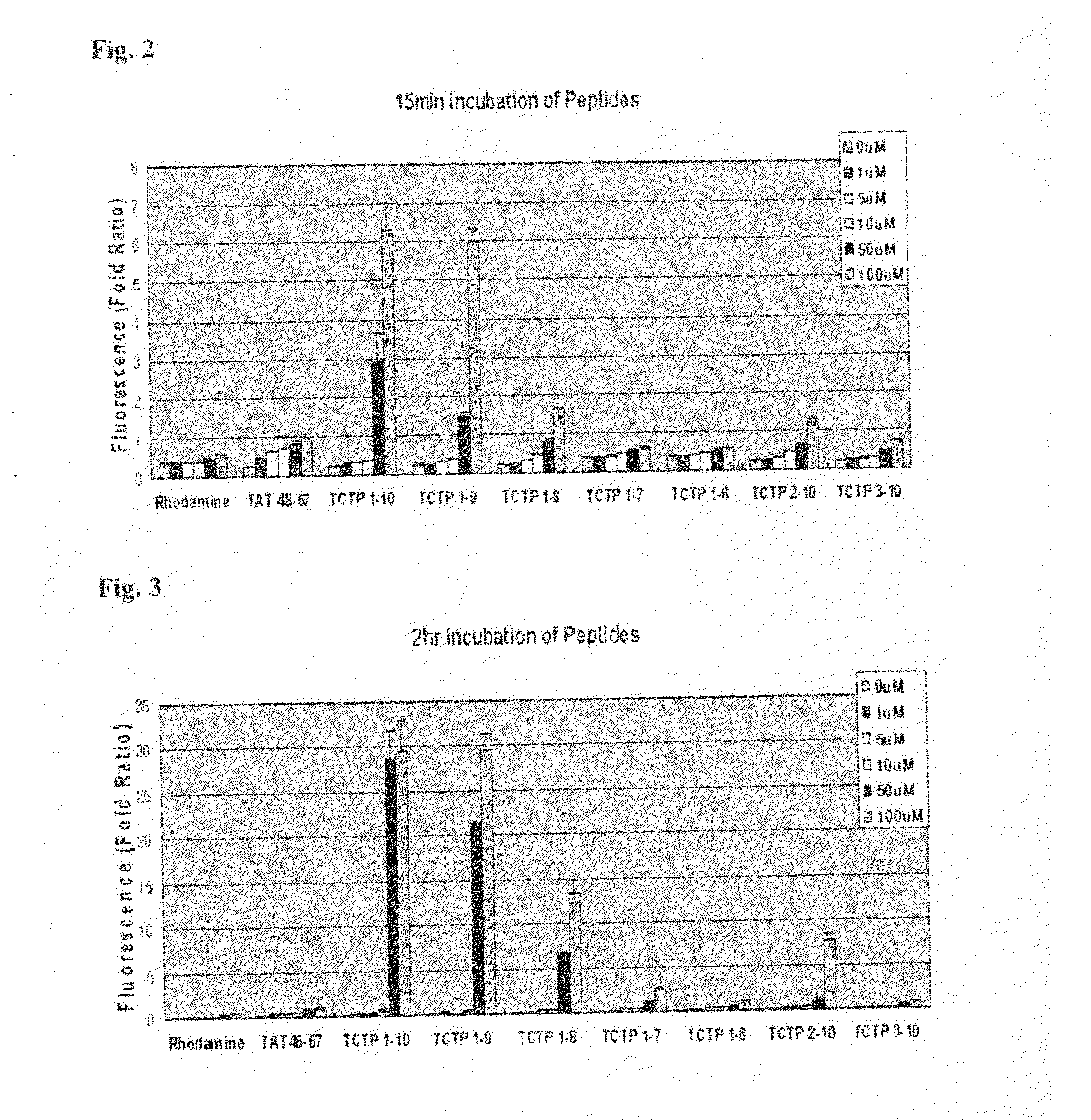
[0112]As shown in Example 1, in order to confirm that the N-terminus of TCTP can function as a PTD, the peptides consisting of N-terminus of TCTP were constructed and examined for cell penetrating efficiency.
1) Synthesis of Various Peptides Corresponding N-Terminus Amino Acid of TCTP
[0113]TCTP-derived peptides and control peptide, TAT 48-57 were synthesized as follow.
Sequence ofClassificationamino acidSEQ ID No.Residues of TCTP(1-10)MIIYRDLISH1Residues of TCTP(1-9)MIIYRDLIS2Residues of TCTP(1-8)MIIYRDLI3Residues of TCTP(2-10)IIYRDLISH4Residues of TCTP(1-7)MIIYRDL5Residues of TCTP(1-6)MIIYRD6Residues of TCTP(3-10)IYRDLISH7Control TAT(48-57)GRKKRRQRRR19
[0114]N-terminus of each peptides was labeled with fluorescence dye, rhodamine and C-terminus was protected. Peptide purity (>95%) was determined by HPLC. Syntersis of the peptides was requested to PEPTRON, Inc.
[0115]Negative control was a fluorescence dy...
example 3
Identification of Intracellular Translocation of TCTP-Derived Peptide by Fluorescence Microscope
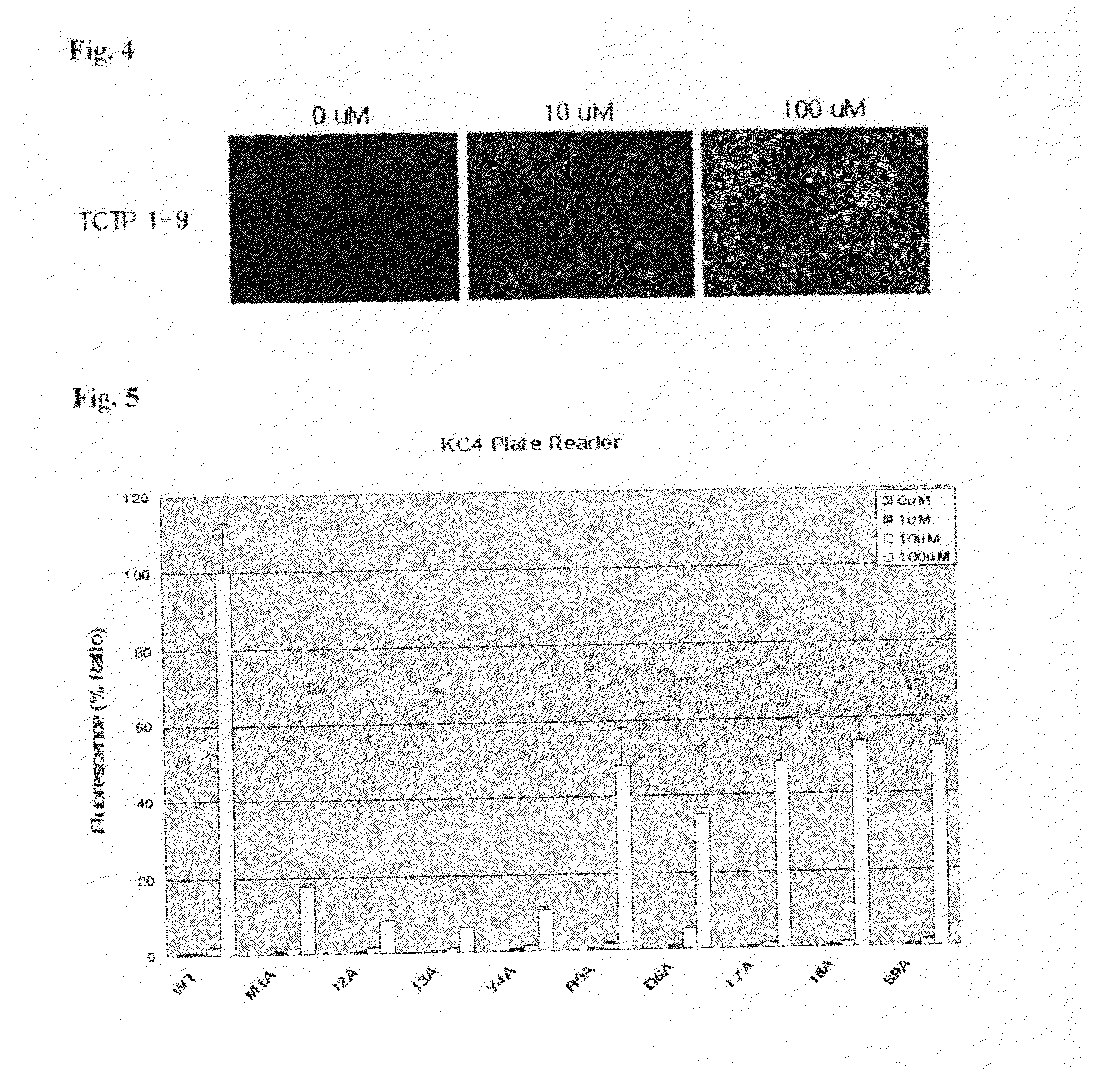
[0126]The intracellular translocation of the peptide was identified by fluorescence microscope. HeLa cells were treated with TCTP (1-9)(SEQ ID No.: 2) at a concentration of 10 μM and 100 μM by the same method of Example 2-2). A point of difference was that HeLa cells were seeded in 12 well-plate covered a glass since the plastic plate had a property of fluorescence interference. After washing, cells on cover glass attached slide glass were observed (FIG. 4).
[0127]As shown in FIG. 4, the peptide of the present invention was weakly translocated at a low concentration of 10 μM and strongly at a high concentration of 100 μM. It was found that the peptides were distributed widely in cytoplasm and nucleus of the cell.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| basic | aaaaa | aaaaa |
| confocal microscope | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com