Method for effectively inactivating parvovirus in prothrombin complex and preparation obtained by method
A technology of prothrombin and parvovirus, applied in medical preparations containing active ingredients, blood diseases, extracellular fluid diseases, etc., can solve the problem of not being able to guarantee the safety of heat-resistant non-lipid enveloped viruses and the difficulty of ensuring product potency Recovery rate and other issues to achieve the effects of reduced production costs, high safety, and shortened time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 Preparation of prothrombin complex preparation of the present invention
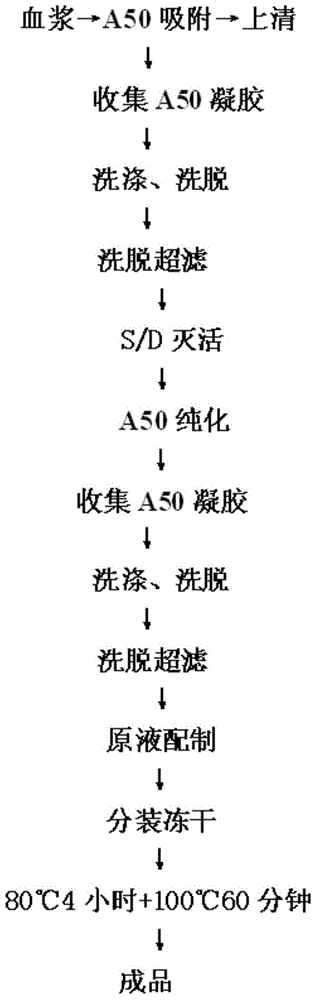
[0029] like figure 1 Shown schematic diagram, preparation of thrombin complex preparation of the present invention:
[0030] 1. Preparation of thrombin complex solution
[0031] Ⅰ. Separation and purification
[0032] Collect the A50 gel after absorbing plasma, wash the gel with 3-10 times the amount of gel washing solution (solution composition: 0.1-0.3M NaCl, 0.01-0.03M sodium citrate, pH: 6.5-7.5), and then Use 3-10 times the amount of gel eluent (recipe: 0.01-0.03M sodium citrate + 1M-2M sodium chloride, pH 6.5-7.5) to elute the product, collect the eluted protein solution for ultrasonography filter.
[0033] II. S / D inactivation
[0034] Slowly add 11% S / D concentrated solution (formula: 11% Tween-80 + 3.3% tributyl phosphate) into the protein concentrated solution, and add while stirring, so that the final concentration of Tween 80 is 0.8-1.2%, and the final concentration o...
Embodiment 2
[0044] Embodiment 2 Preparation of prothrombin complex preparation of the present invention
[0045] like figure 1 Shown schematic diagram, preparation of thrombin complex preparation of the present invention:
[0046] 1. Preparation of thrombin complex solution
[0047] With embodiment 1.
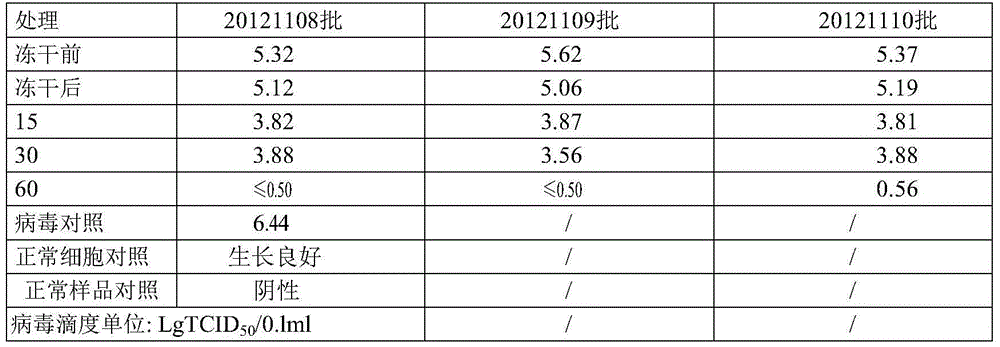
[0048] 2. Dry heat inactivation
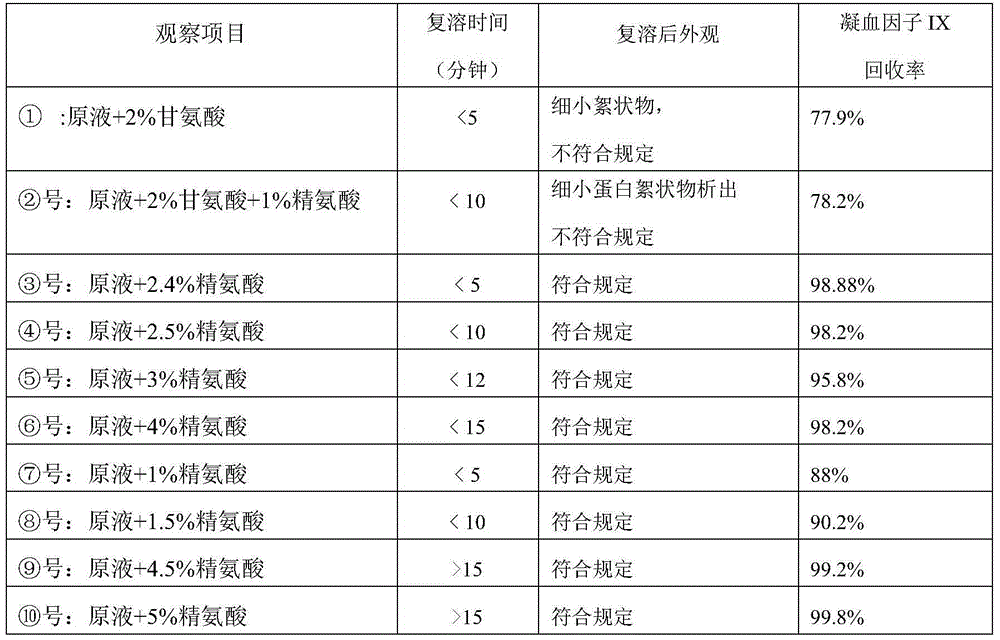
[0049] (1) Take the prothrombin complex prepared in step I, add 3% (w / v) arginine, sterilize, subpackage, and freeze-dry;
[0050] (2) Dry heat treatment: treat at 80°C for 10 hours, and then at 100°C for 30 minutes to obtain the prothrombin complex preparation of the present invention.
Embodiment 3
[0051] Embodiment 3 Preparation of prothrombin complex preparation of the present invention
[0052] 1. Preparation method
[0053] 1. Preparation of thrombin complex solution
[0054] With embodiment 1.
[0055] 2. Dry heat inactivation
[0056] (1) Take the prothrombin complex prepared in step I, add 4% (w / v) arginine hydrochloride, sterilize, subpackage, and freeze-dry;
[0057] (2) Dry heat treatment: treat at 80°C for 2 hours, and then at 100°C for 60 minutes to obtain the prothrombin complex preparation of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com