Preparation method of human thrombin
A technology of human thrombin and prothrombin, applied in biochemical equipment and methods, enzymes, peptidases, etc., can solve problems such as the inability to guarantee the long-term stability of thrombin activity and the impact of thrombin activity, and achieve obvious practical significance , good long-term stability and high vitality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. 80 kilograms of component III obtained by the ethanol fractionation method of 2.3 tons of healthy human plasma obtained 11.5 kilograms of prothrombin complex solution through SD virus inactivation and ion exchange resin separation and purification (see the prothrombin complex virus for specific methods. Research on inactivation [J]. Chinese Journal of Biological Products, 1995, 8 (3): 101-104; Virus inactivation and verification of human prothrombin complex [J]. Chinese Journal of Blood Transfusion, 1998, 11 (4 ):195-197). After 10mM calcium ion activation, when the detected thrombin activity is greater than 1000IU / ml and tends to be stable, the calcium ion incubation is stopped, and the cation exchange resin is used to separate and purify thrombin. The chromatographic medium is SP-Sephadex C50, the equilibrium buffer is 0.1M NaCl, 0.02M Tris-HCL, pH6.5 solution, and the elution buffer is 0.5M NaCl, 0.02M Tris-HCL, pH6. 5 solution. The eluent was 15.2 kg of thrombi...
Embodiment 2
[0040] 1. 81 kilograms of component III obtained by ethanol fractionation of 2.3 tons of healthy human plasma obtained 12 kilograms of prothrombin complex solution through SD virus inactivation and ion exchange resin separation and purification (see the prothrombin complex virus for specific methods. Research on inactivation [J]. Chinese Journal of Biological Products, 1995, 8 (3): 101-104; Virus inactivation and verification of human prothrombin complex [J]. Chinese Journal of Blood Transfusion, 1998, 11 (4 ):195-197). After 100mM calcium ion activation, when the detected thrombin activity is greater than 1000IU / ml and tends to be stable, the calcium ion incubation is stopped, and the cation exchange resin is used to separate and purify thrombin. The chromatography medium is SP-Sepharose FF, the equilibration buffer is 0.15M NaCl, 0.01M Tris-HCL, pH7.0 solution, and the elution buffer is 1.0M NaCl, 0.01M Tris-HCL, pH7. 0 solution. Obtained 15.4 kg of thrombin eluate with a ...
Embodiment 3
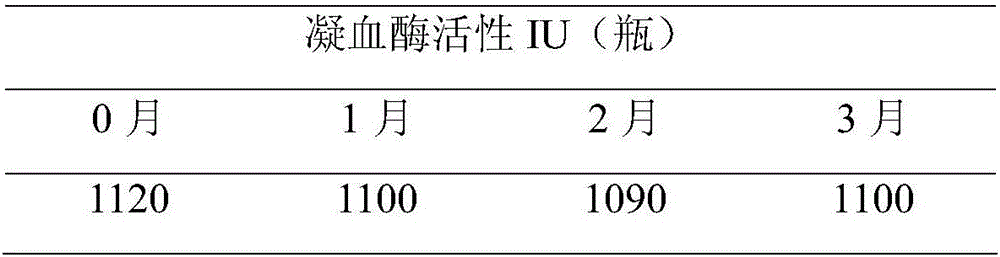
[0044] Place the finished thrombin product prepared in Example 2 at 25°C, take out samples at 0, 1, 2, and 3 months respectively, redissolve with 2ml of water, detect thrombin activity, and observe the stability of thrombin. The results are shown in the following table :
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com