Method for producing human prothrombin complex
A technology of human prothrombin and production method, which is applied in the fields of biochemical equipment and methods, enzymes, blood diseases, etc. The effect of stability, increasing yield, and ensuring virus safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
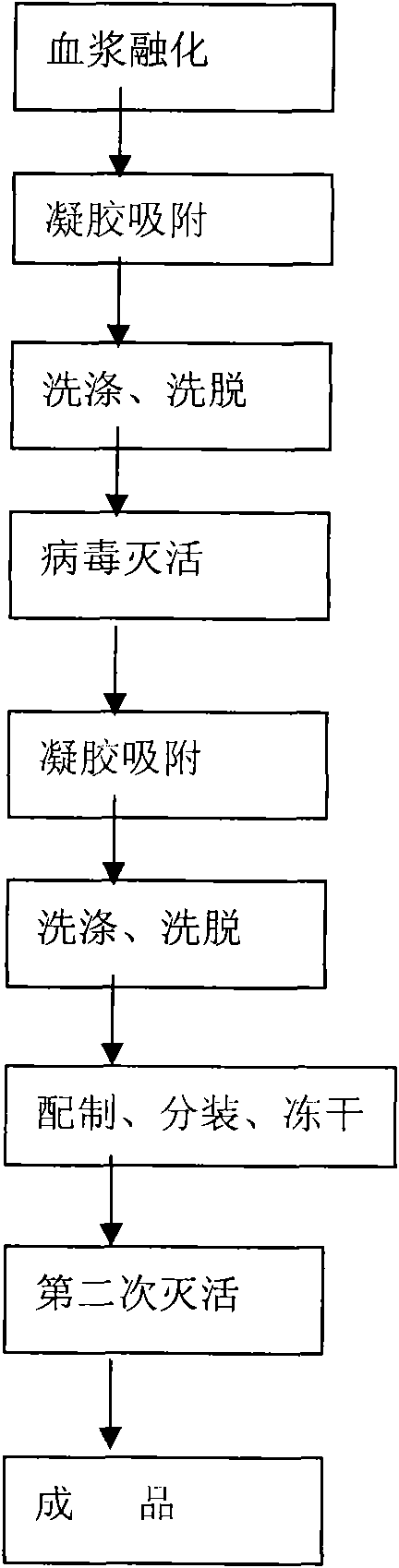
[0022] A. Separation and extraction
[0023] Melt the raw plasma, keep the plasma temperature at 0°C, and centrifuge at 0°C to remove the cryoprecipitate, heat the supernatant from the cryoprecipitate to 12°C, add 1% swollen diethylaminoethyl cross-linked agarose gel and stir , adsorbed for 45 minutes, collected the gel, quickly rinsed the gel 3 times with the washing solution, then soaked in the eluent for 10 minutes / time, washed 3 times in total, eluted the product, collected the eluted protein solution for ultrafiltration, Pre-concentrate, then use dialysate ultrafiltration to make the residual sodium chloride concentration less than 0.85% (g / ml), concentrate the product until the titer of factor IX is about 10IU / ml, and measure the weight of the concentrate;
[0024] B. Virus inactivation
[0025] While stirring the concentrated solution, slowly add 11% S / D solution in a ratio of 10:1, so that the final concentration of polysorbate-80 is 1% (g / ml), and the final concentra...
Embodiment 2
[0040] A. Separation and extraction
[0041] Melt the raw plasma, keep the plasma temperature at 2°C, and centrifuge at 2°C to remove the cryoprecipitate, heat the supernatant from the cryoprecipitate to 13°C, add 1% swollen diethylaminoethyl cross-linked agarose gel and stir , adsorbed for 45 minutes, collected the gel, quickly rinsed the gel 3 times with the washing solution, then soaked in the eluent for 10 minutes / time, washed 3 times in total, eluted the product, collected the eluted protein solution for ultrafiltration, Pre-concentrate, then use dialysate ultrafiltration to make the residual sodium chloride concentration less than 0.85% (g / ml), concentrate the product until the titer of factor IX is about 10IU / ml, and measure the weight of the concentrate;
[0042] B. Virus inactivation
[0043] While stirring the concentrated solution, slowly add 11% S / D solution in a ratio of 10:1, so that the final concentration of polysorbate-80 is 1% (g / ml), and the final concentra...
Embodiment 3
[0057] A. Separation and extraction
[0058] Thaw the raw plasma, keep the plasma temperature at 4°C, and centrifuge at 4°C to remove cryoprecipitate, remove the cryoprecipitate supernatant and raise the temperature to 14°C, add 1% swollen diethylaminoethyl cross-linked dextran gel Stir, absorb for 45 minutes, collect the gel, quickly rinse the gel with washing solution for 3 times, then soak in the eluent for 10 minutes / time, wash 3 times in total, elute the product, collect the eluted protein solution for ultrafiltration , pre-concentration, and then use dialysate ultrafiltration to make the residual sodium chloride concentration less than 0.85% (g / ml), and the product is concentrated to about 10IU / ml in factor IX titer, and the weight of the concentrated solution is measured;
[0059] B. Virus inactivation
[0060] While stirring the concentrated solution, slowly add 11% S / D solution in a ratio of 10:1, so that the final concentration of polysorbate-80 is 1% (g / ml), and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com