Pichia yeast expressing recombinant human blood coagulation factor VII, and preparation and use thereof
A human blood coagulation factor and blood coagulation technology, applied in the field of constructs for transforming yeast cells, can solve problems such as low yield, changing protein immunogenicity, shortening residence time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0088] The preparation method of the expression cassette of the present invention comprises the following steps:
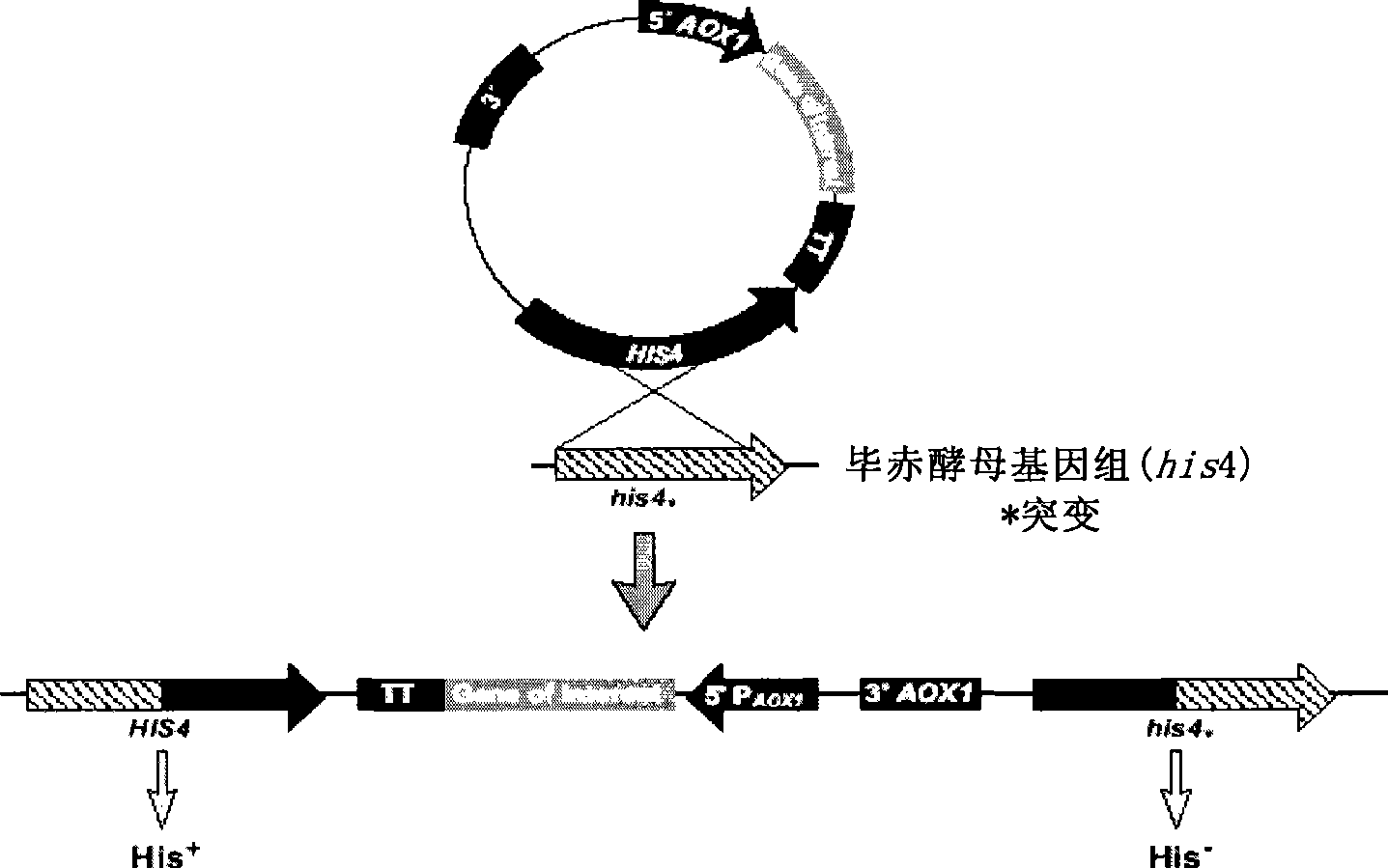
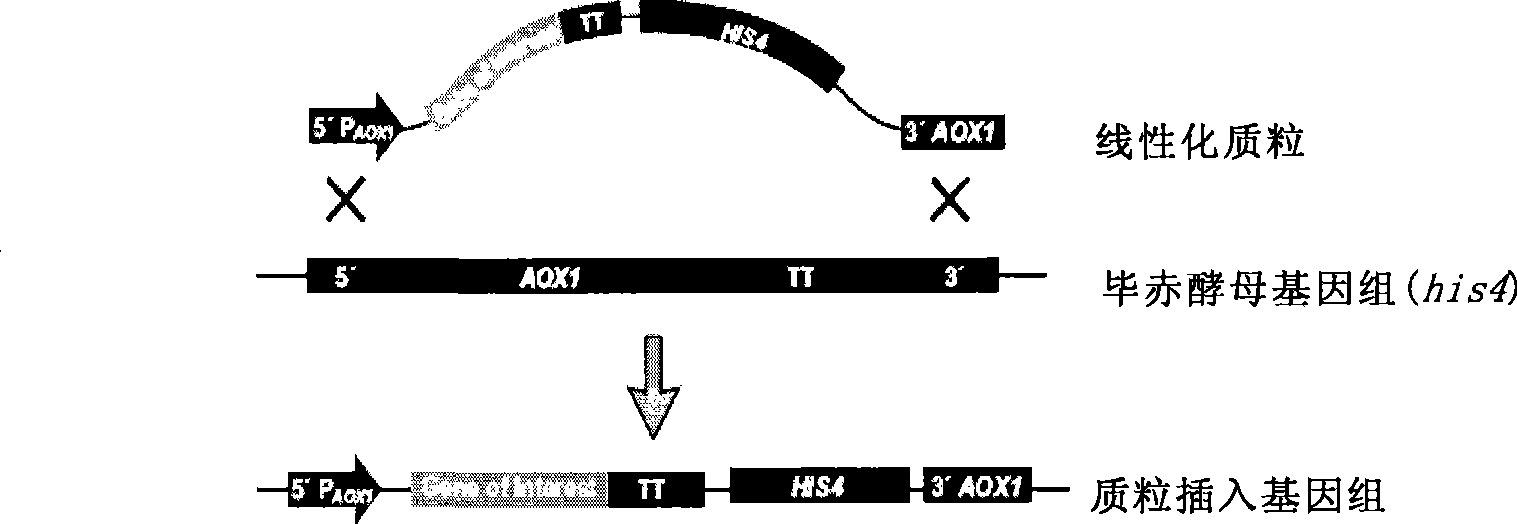
[0089] (1) site-directed insertion of the human coagulation factor VII gene into a plasmid comprising the start signal element AOX and the stop signal element AOX (TT) to obtain a single-copy expression plasmid;
[0090] (2) Transforming the single-copy expression plasmid to obtain a complete human coagulation factor VII expression cassette.
[0091] In a preferred embodiment of the present invention, the plasmid used can be: pPIC9K, pPIC3.5K, pAO815 or pHIL-S1, etc., as long as it contains the start signal element AOX and the stop signal element AOX (TT); pPIC9K plasmid is preferred.
[0092] The human blood coagulation factor VII gene can be inserted between the 5'AOX1 and 3'AOX(TT) of the plasmid, preferably between the BamHI restriction site and the EcoR1 restriction site.
[0093] Conventional methods such as PCR can be used to amplify the FVII gene with the...
Embodiment 1
[0115] Example 1. Secretion and expression of recombinant human coagulation factor VII with different N-terminal primary structures in different strains of Pichia pastoris
[0116] 1. PCR amplification of FVII target gene
[0117] 1.1 Primer design
[0118] Primers were designed with reference to the full gene sequence of human blood coagulation factor VII (SEQ ID NO: 1), and synthesized by Shanghai Sangong.
[0119] Primer 1 5′-GC CTCGAG AAAAGAGCCAACGCGTTCCTGGAGGAG-3' (SEQ ID NO: 3)
[0120] wxya
[0121] Primer 25′-GC CTCGAG AAAAGA GAGGCTGAAGCT GCCAACGCGTTC-3' (SEQ ID NO: 4)
[0122] XhoI GluAlaGluAla
[0123] Primer 35′-GC GAATTC GCTGCTGGGCTAGGGAAATGG-3' (SEQ ID NO: 5)
[0124] EcoRI
[0125] 1.2 PCR reaction
[0126] Use P1, P3 primers to carry out PCR, and amplify to obtain pPIC9K / FVII target gene (excluding Glu-Ala repeat sequence); use P2, P3 primer to amplify to obtain pPIC9K / Glu-Ala-Glu-Ala-FVII target gene (containing...
Embodiment 2
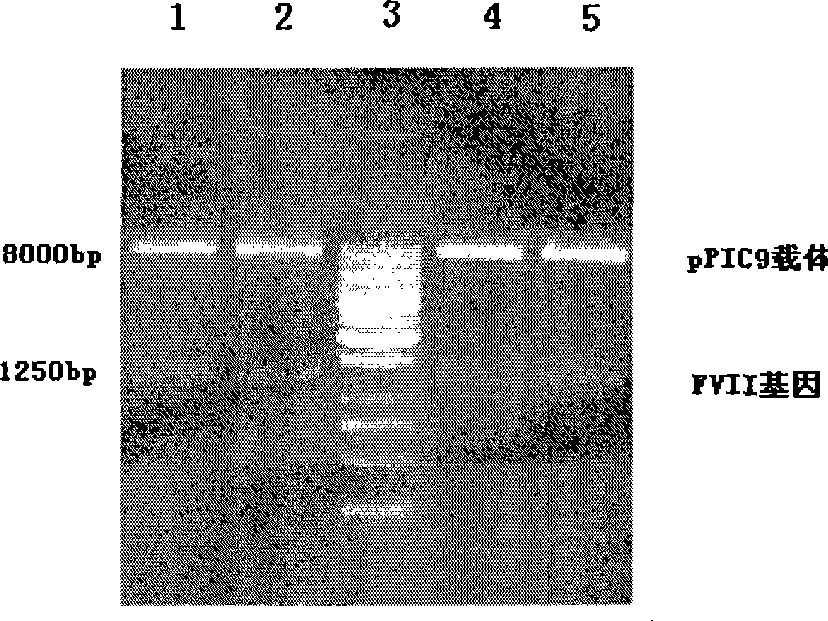
[0174] Example 2. Construction and enzyme digestion identification of human coagulation factor VII high copy recombinant
[0175] The method of repeating and cascading the complete expression unit is used to construct the multi-copy recombinant of recombinant FVII. In this study, the special properties of two isotail enzymes BamHI:G↓GATCC and BglII:A↓GATCT at both ends of the complete expression element were used to construct high-copy recombinants.
[0176]After digesting the single-copy recombinants constructed in Example 1 with BamHI and BglII enzymes, the target fragments were recovered, and the insert fragments were self-ligated by using the properties of the homologous enzymes, and then treated with BamHI and BglII enzymes. connection system. If the expression unit is forward repeating series, the homologous enzyme at the junction of the two units will not become any restriction enzyme recognition site after joining, and the forward junction structure will be preserved;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com