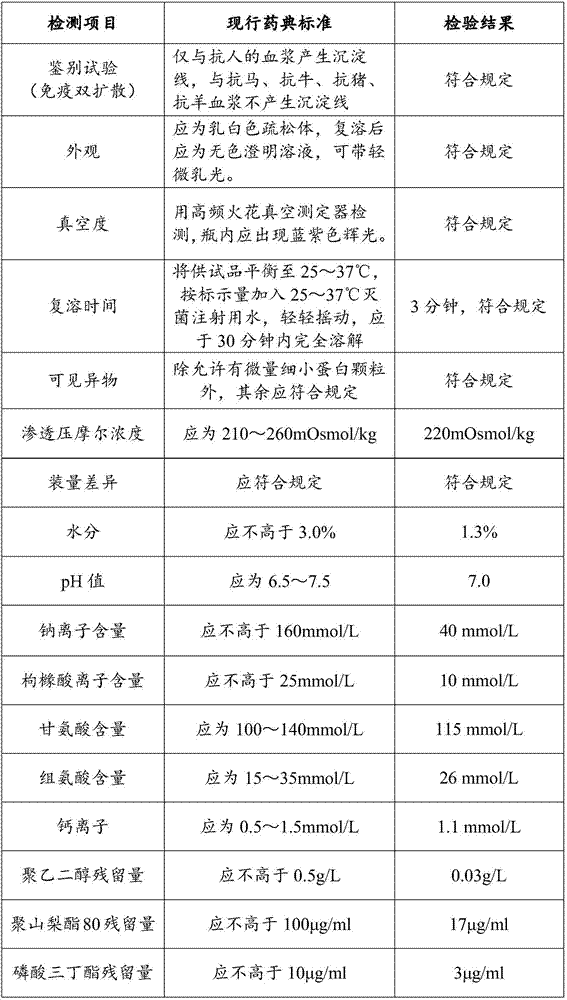
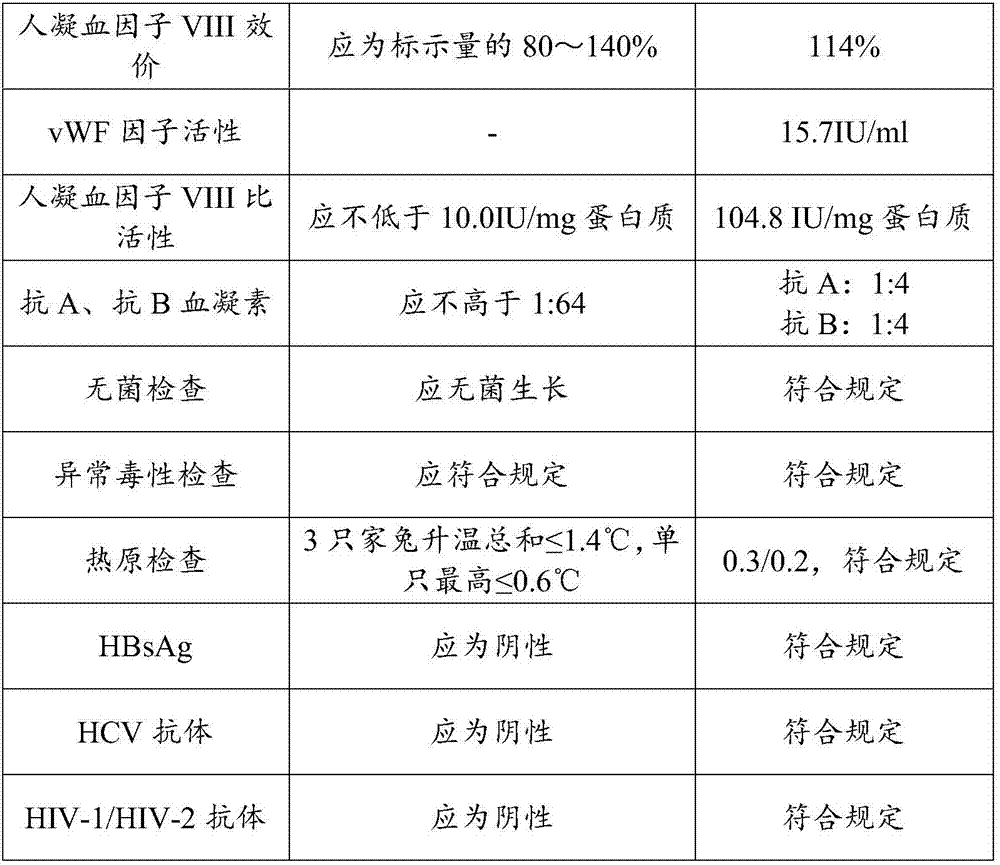
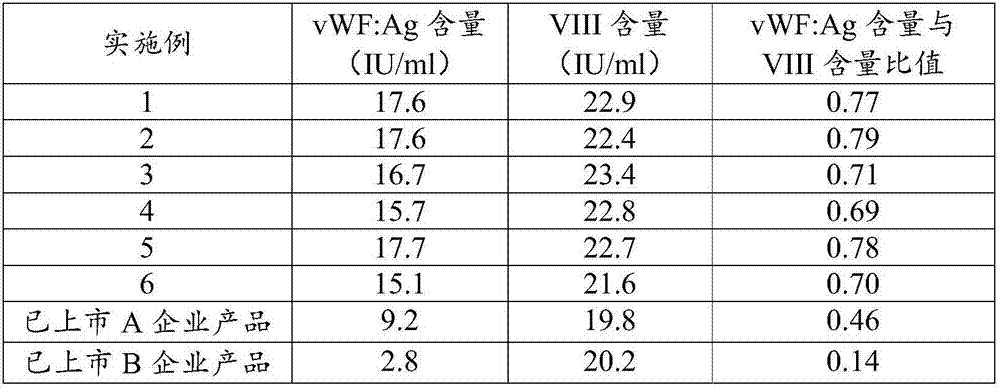
Preparation method of human blood coagulation factor VIII and human blood coagulation factor VIII product
A technology of human blood coagulation factor and cryoprecipitation, which is applied to the preparation method of factor VII, peptide, coagulation/fibrinolysis factor, etc. It can solve the problems of low specific activity, low yield, and failure to meet the basic medication needs of patients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] The method for preparing human coagulation factor VIII provided in this embodiment includes the following steps:
[0070] 1. Steps for collecting raw plasma:
[0071] 1.1 The collection and quality of plasma should comply with the provisions of "Human Plasma for the Production of Blood Products" in the current edition of the Pharmacopoeia of the People's Republic of China. It should be frozen within 4 hours after collection and stored at -30°C for no more than 1 year.
[0072] 1.2 The plasma should have no clots, no fibrin precipitation, no lipemia, and no hemolysis.
[0073] 2. Cryogenic precipitation preparation steps:
[0074] Weigh 2097kg of frozen plasma (total 3495 human plasma), soak or wipe the surface with 70% ethanol solution (the ethanol solution is required to control 70%-75%), then break the bag and combine it into the tank. Melt in a water bath, and control the melting temperature at 4°C (control the melting temperature at 0-4°C).
[0075] Use a low-temperature high...
Embodiment 2
[0107] The method for preparing human coagulation factor VIII provided in this embodiment is basically the same as that in Embodiment 1, except that in this embodiment:
[0108] The balance solution contains: tromethamine 18mmol / L, sodium chloride 90mmol / L, glycine 110mmol / L and calcium chloride 38μmol / L.
[0109] The dialysate contains: sodium nitrate 11mmol / L, histidine 28mmol / L, glycine 130mmol / L and calcium chloride 1.1mmol / L.
Embodiment 3
[0111] The method for preparing human coagulation factor VIII provided in this embodiment is basically the same as that in Embodiment 1, except that in this embodiment:
[0112] The balance solution contains: tromethamine 22mmol / L, sodium chloride 110mmol / L, glycine 130mmol / L, and calcium chloride 42μmol / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com