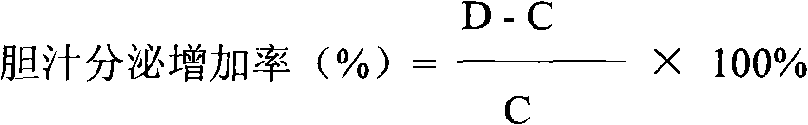Patents
Literature
257results about How to "Reduce denaturation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
High pressure spray-dry of bioactive materials
ActiveUS7378110B2Reduce denaturationReduce shear stressBiocidePowder deliveryHigh concentrationHigh pressure
This invention provides compositions and methods providing, e.g., stable powder particles containing bioactive materials. The methods include, e.g., high pressure spraying of the bioactive materials in solution or suspension, with viscosity enhancing agents and / or surfactants. Compositions of the invention provide, e.g., high initial purity, high stability in storage, and reconstitution at high concentrations.
Owner:MEDIMMUNE LLC
Drug delivery system and preparation method thereof
InactiveCN101485629AHigh feasibilityImprove stabilityLyophilised deliveryEmulsion deliveryOrganic solventSide effect
The invention belongs to the technical field of medicines, and discloses a delivery system based on protein-phospholipid. The system comprises a protein-phospholipid dispersion with the weight ratio of between 1:100 and 100:1, wherein the grain size of the protein-phospholipid is between 5 and 1,000nm, and the surface of the phospholipid or the surface and the inside of the phospholipid are coated / covered by a protein layer. The preparation method comprises: preparing a phospholipid containing phase, preparing a protein containing water phase, performing the homogenization treatment on the phospholipid containing phase and the protein containing water phase at a temperature of between 0 and 40 DEG C and under a pressure of between 400 and 40,000psi, and obtaining a dispersion system with the average grain size of less than 1,000nm. The delivery system has the advantages of realizing the delivery in various ways such as injection, oral taking, cavities and canals, skin and the like, and lowering side effects of medicines. The delivery system solves the problems of drug-loading rate and stability of protein nano-grain and liposome, and adopts low-toxicity organic solvent, even no organic solvent; and the preparation method is easy for industrialized application, and can prepare a liquid type protein-phospholipid containing delivery system.
Owner:SHENYANG PHARMA UNIVERSITY
Drug Delivery System, its Preparation Process and Use
InactiveUS20110064794A1Improve freeze-thaw stabilityExpanded drug loading categoryBiocideNanomedicineMedicinePhospholipid
A protein-phospholipid dispersion preparation in a drug delivery system is provided, in which the weight ratio of protein to phospholipid is 1:300-300:1 and the particle size is between 5 nm and 1000 nm. The preparation process of the said preparation contains the mixture of protein phase and phospholipid phase and the homogenization process. The said drug delivery system can be used in many different pharmaceutical agents.
Owner:SHENYANG PHARMA UNIVERSITY +1
Nanocoating for improving biocompatibility of medical implants
InactiveUS20050084513A1Prevent denaturationPrevent protein denaturationPowder deliveryMicroencapsulation basedImplant surfaceBiocompatibility Testing
A coating for an implant surface comprising one or more nanoparticles of less than or equal to 500 nanometers and an implant surface capable of receiving the nanoparticles, the implant selected from the group consisting of metal, carbon, graphite, polymer, protein, nucleic acid, microorganisms, hydrogel, liquid, porous and polymer blend particles, and combinations thereof. The coating promotes characteristics on the implant surface such as reducing protein unfolding, preventing inflammatory and fibrotic cell accumulation, reducing the number of such cell attachment sites and preventing other adverse biological reactions. The coating may be applied on any material via physical and / or chemical binding. The coating may further comprise a surfactant and may include a tag, adsorbed, absorbed or incorporated onto the nanoparticle. The coating on an implant surface is used for purposes that may be cosmetic, therapeutic, preventative, reconstructive, monitoring and replacement. The coating may also be used for in vitro purposes.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
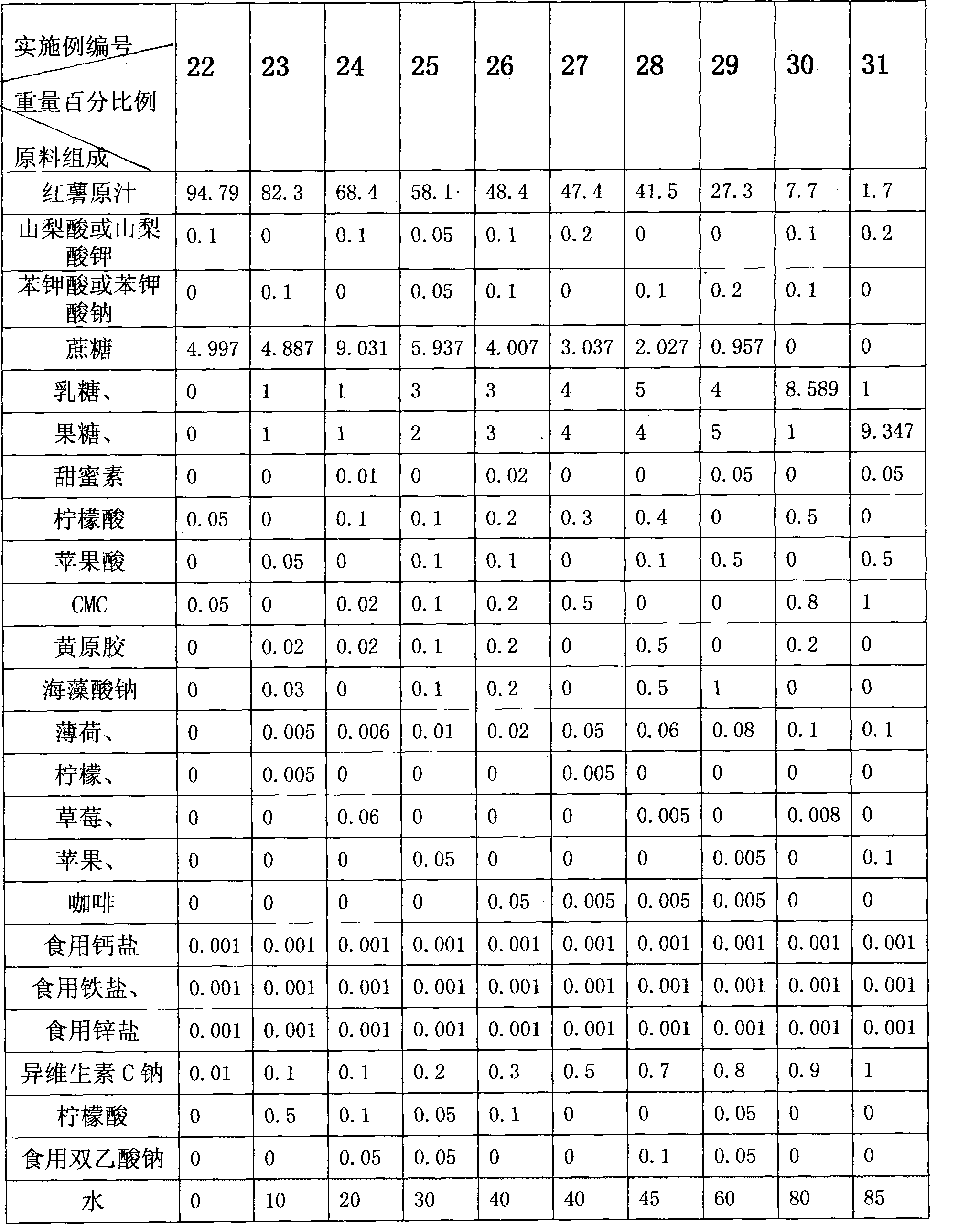
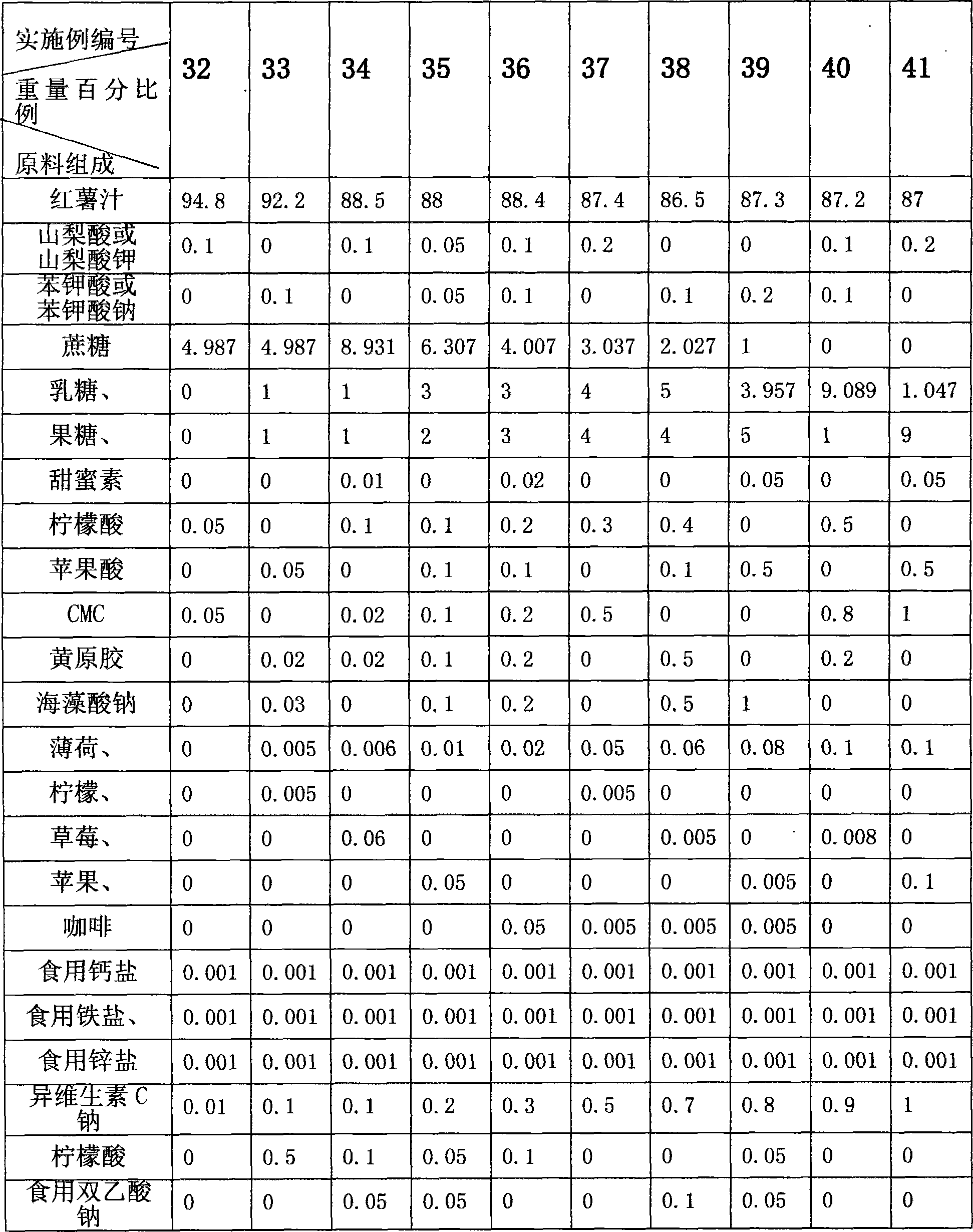
Method for processing sweet potato beverage
ActiveCN101243895AShorten the processing cycleHigh utilization rate of raw materialsFood preparationChemistryColloid mill
The invention discloses a making method for sweet potato beverage, which is characterized in that: the making method comprises the following steps: 1) rinsing: fresh sweet potatoes are manually or mechanically rinsed, to remove mud, sand and impurity; 2) crushing and grinding juice: the cleaned fresh sweet potatoes are grinded until the grains are less than 1000 micron through a colloid mill or apulping machine, obtaining sweet potato juice. Preservative, sweetener, acidity agent, stabilizing agent, essence, vitamin or one, two or more of the minerals can also be added into the sweet potato juice. The making method has the advantage of simple technology, low production cost, good product quality, high nutritional value, easy absorption and utilization for human body, ability of liquid, solid, grain, capsule, buccal tablet or tablets product forms; and the products made by the method has the advantages that: the dietary fiber, vitamin, protein, polysaccharide, anthocyanin and other compositions of the material are remained, nutritional ingredient of sweet potatoes and active components which can improve humane immunocompetence are remained to the utmost extent, and is an excellentgreen natural health beverage.
Owner:SICHUAN GUANGYOU SWEET POTATO & FOOD PROD CO LTD
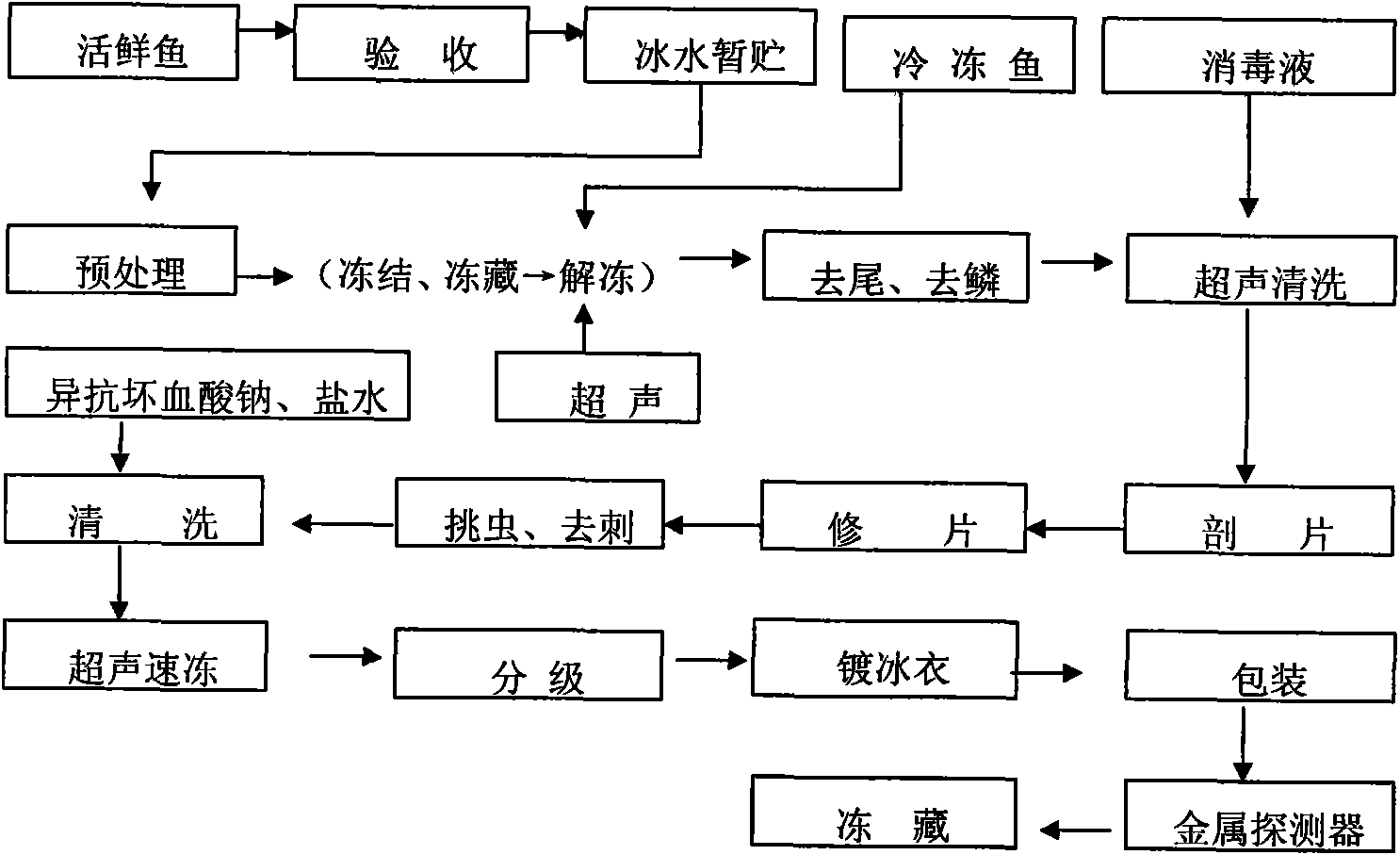
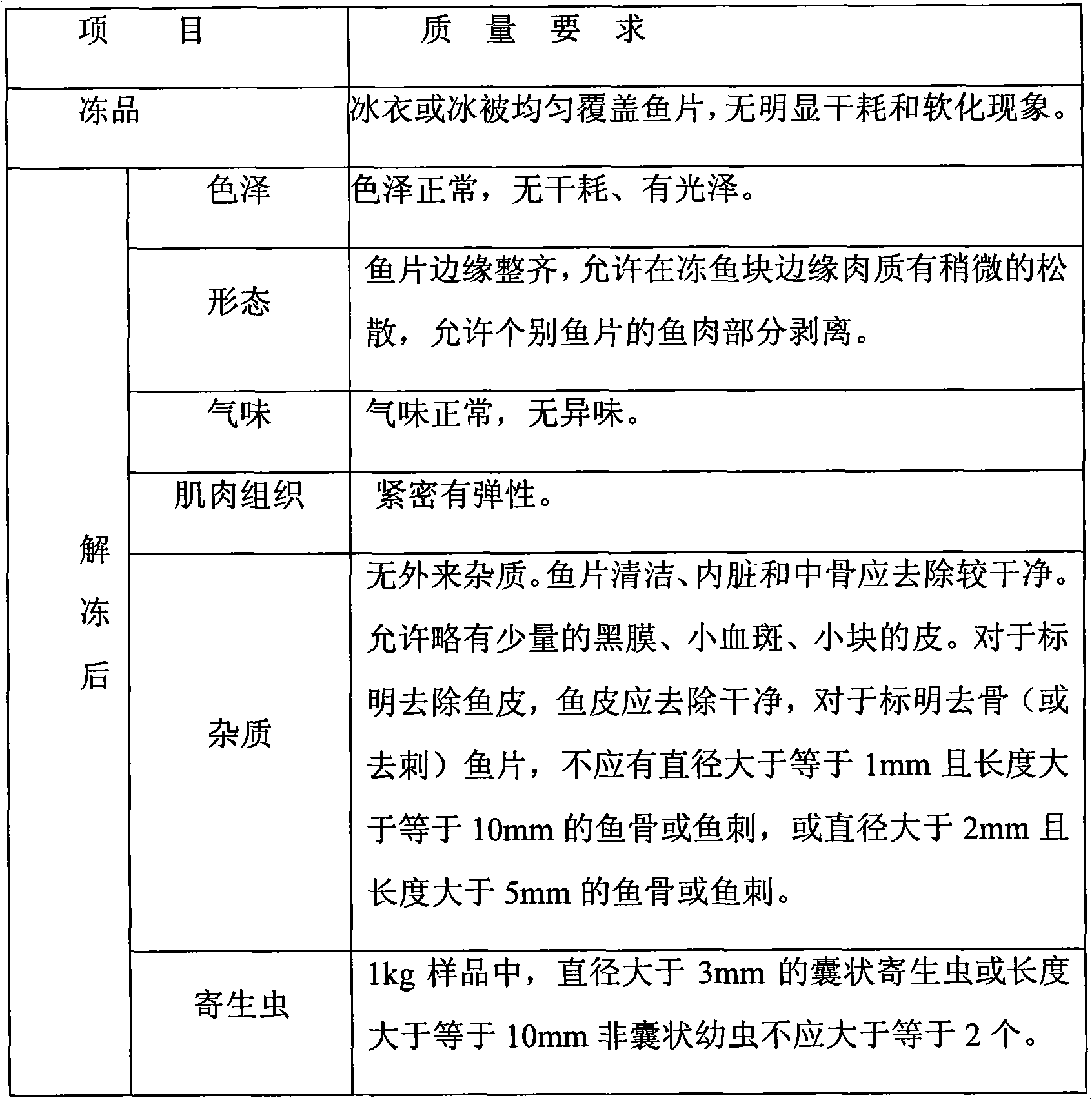
Processing method of sushi sashimi
InactiveCN101919539AExtended shelf lifeAvoid "cooking"Fish washing/descalingMeat/fish preservation by freezing/coolingIce waterAquatic product
The invention provides a processing method of sushi sashimi, which belongs to the field of processing of aquatic products. The processing method comprises the steps of taking a live fresh scaly fish as a raw material, carrying out acceptance, temporary storage in ice water and pretreatment, or taking a frozen scaly fish as the raw material, firstly carrying out ultrasonic thawing, further removing a tail, removing scales, carrying out ultrasonic cleaning, cutting into slices, trimming, picking out insects, removing bones, cleaning, carrying out ultrasonic quick freezing, classifying, glazing, packaging, carrying out metal detection, freezing, and carrying out other process steps for processing the sushi sashimi. The sushi sashimi processed by adopting the method has good quality, and the sensory, the physical and the chemical properties, as well as the safety sanitation quality are in line with requirements; furthermore, the processing method has short thawing time, short freezing time and high processing efficiency, and can be popularized in the processing of the aquatic products.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Protein purification
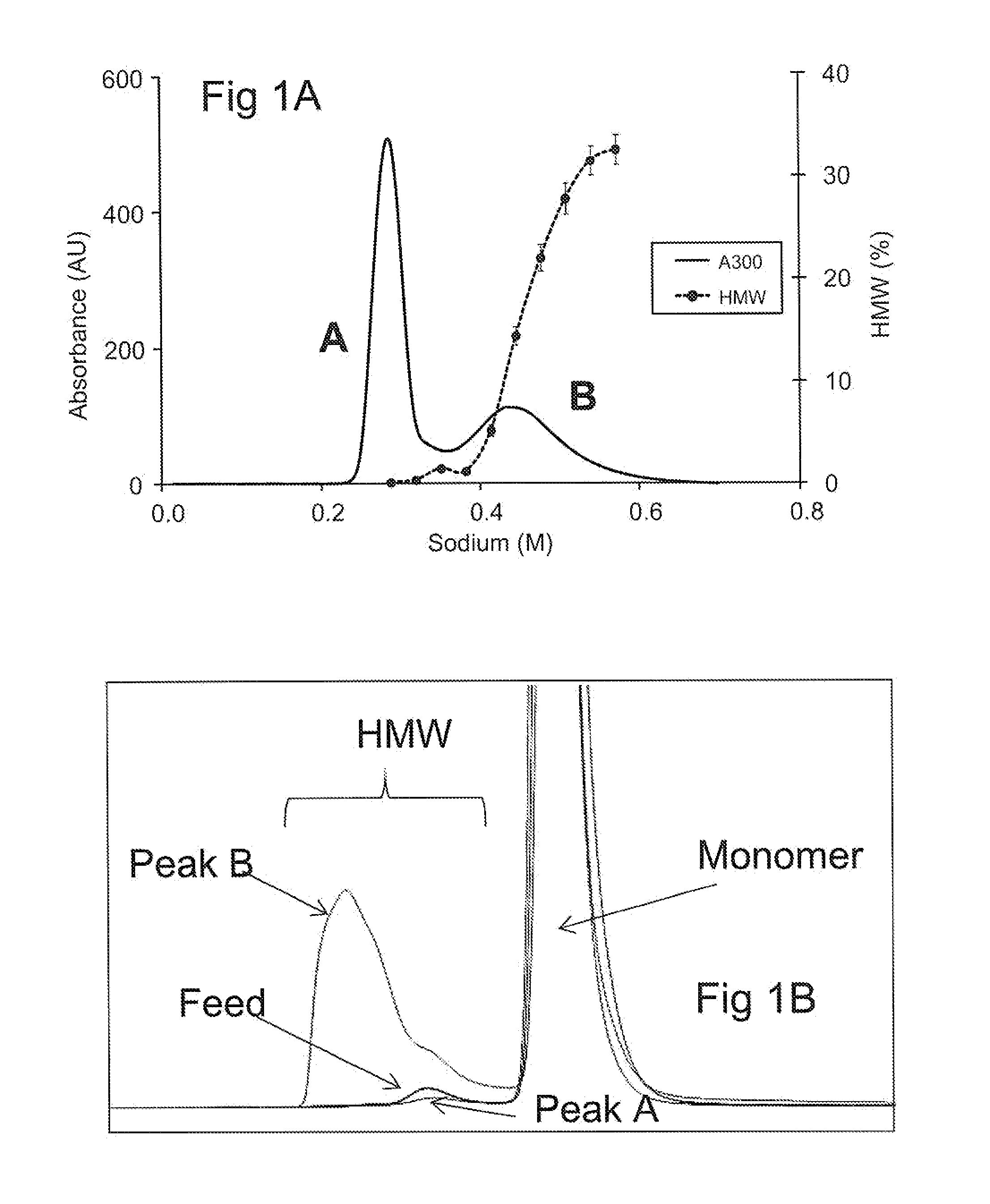
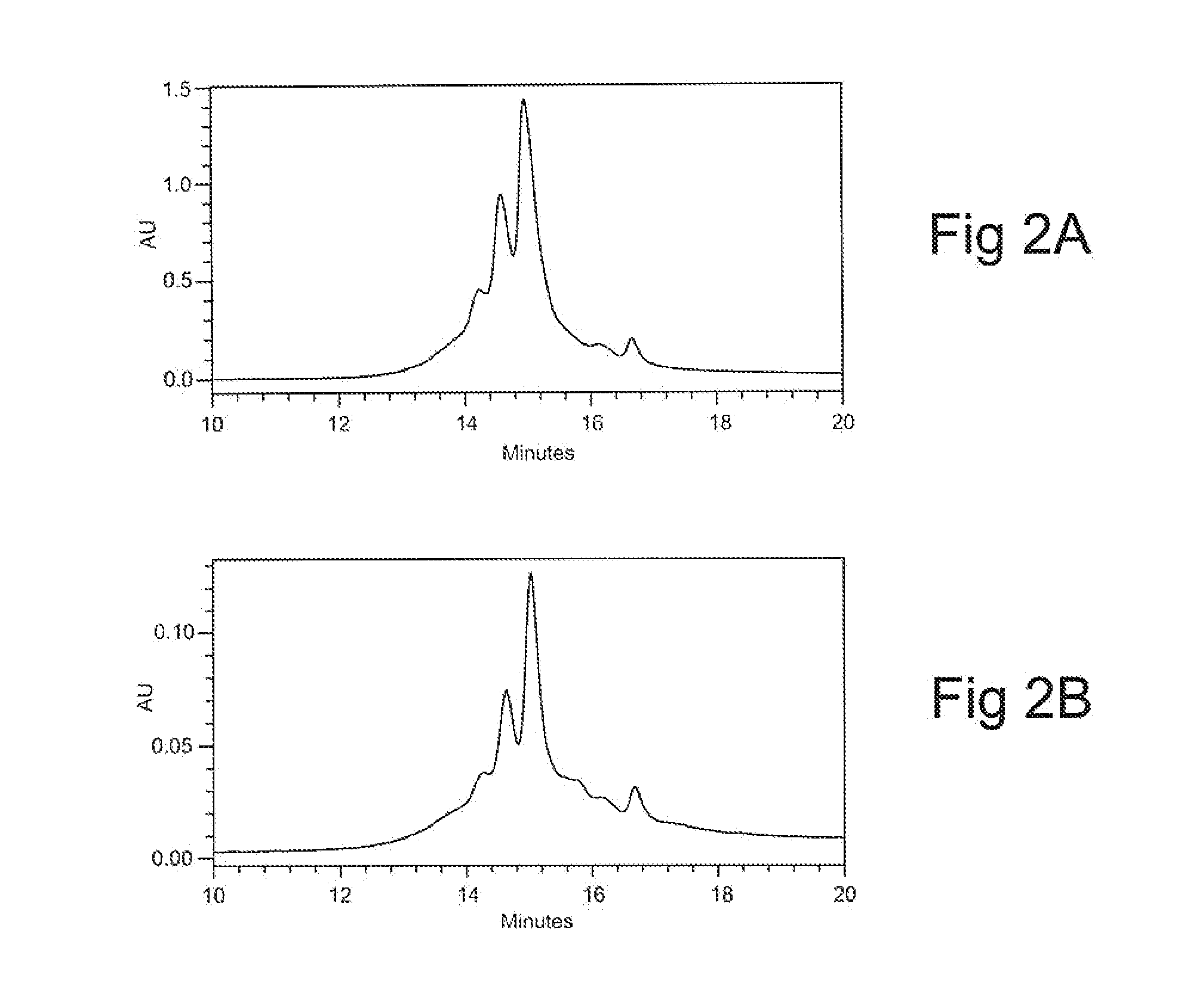
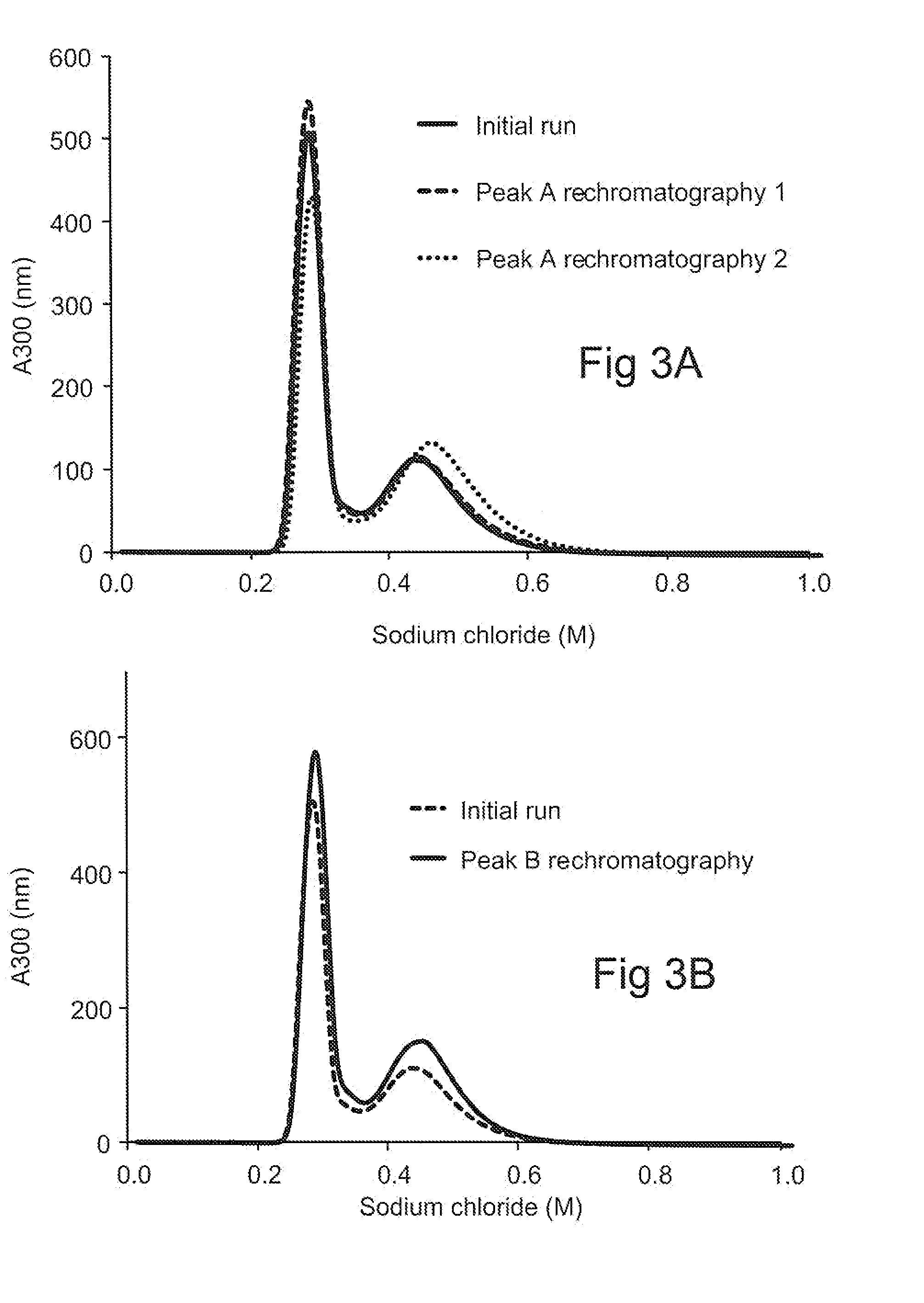
InactiveUS20120149878A1Reducing high molecular weight specie (HMW) formationReduces HMW formationPeptide preparation methodsDepsipeptidesGlycineOn column
Methods of reducing high molecular weight species (HMW) formation in a sample containing a protein purified using ion exchange (IEX) chromatography are disclosed, as are a number of related methods, e.g., methods of reducing on-column denaturation of a protein in a protein sample purified using an ion exchange (IEX) column or resin. The methods share characteristics of including arginine, glycine and / or histidine in the buffers used during the ion exchange (IEX) chromatography.
Owner:AMGEN INC
Therapeutic composition with a botulinum neurotoxin
ActiveUS7879341B2Low immunogenicityImprove stabilityCosmetic preparationsSenses disorderGenetics manipulationHuman serum albumin
The present invention pertains to pharmaceutical compositions which comprise a botulinum neurotoxin from Clostridium botulinum, the neurotoxin being free of the complexing proteins naturally present in the botulinum neurotoxin complex or being chemically modified or being modified by genetic manipulation. Moreover the pharmaceutical compositions of the instant invention have good stability and are advantageously formulated free of human serum albumin.
Owner:MERZ PHARMA GMBH & CO KGAA
Instant lyophilized fibrinogen and fibrin ferment formulation composition, preparation method and use thereof
ActiveCN101371921ASolve the shortcoming of short storage timeImprove solubilityPeptide/protein ingredientsBlood disorderArginineIrritation
The invention provides a composition of instant freeze-dried fibrinogen and thrombin preparation, a preparation method and a use thereof, and the composition comprises composition 1 composed of 35-70mg / ml of fibrinogen, 9-15mg / ml of sodium citrate, 7-12mg / ml of sodium chloride, 0.3-0.6mg / ml of polysorbate-80, 10-15mg / ml of mannitol, 4 -8mg / ml of arginine and 3.5-5.5mg / ml of glutamic acid by mg / ml and composition 2 composed of 700-1,200 mg / ml of thrombin, 3-6mg / ml of dextran 20, 15-25mg / ml of glycine, 5-7mg / ml of sodium chloride and 4-7mg / ml of calcium chloride by mg / ml. A sealant avoids the risk of spreading of AIDS virus, and the like, increases the drug stability, reduces the degeneration during the virus inactivation process by dry-heat method, protects biological activity, avoids local liquid storage of the using part caused by high permeability and the irritation of a large amount of inorganic salts to tissues, is conductive to the healing of trauma sites and ensures product safety and independent package to the maximum extent, thereby increasing storage time, facilitating use and meeting the clinical and field first-aid needs.
Owner:HANBANG MEDICAL SCI & TECH HARBIN CITY
Pharmaceutical compositions and methods for fabrication of solid masses comprising glucose regulating proteins
ActiveUS20150328287A1Minimize adverse effectsHigh treatment ratePeptide/protein ingredientsMetabolism disorderIntestinal wallsWhite blood cell
Embodiments of the invention provide shaped masses comprising one or more drugs such as proteins or polypeptides and methods for forming such shaped masses. One embodiment provides a shaped mass comprising a drug such as a protein or polypeptide having a biological activity in the body of a mammal. The shaped mass is formed by compression of a precursor material comprising the drug wherein an amount of biologically active drug in the mass is a preserved above a minimum level. Drugs which may be incorporated into the shaped mass may include one or more glucose regulating proteins such as insulin, incretins; and immunoglobulins such as TNF-inhibiting antibodies or interleukin neutralizing antibodies. Embodiments of the shaped mass may be incorporated into a tissue penetrating member which is inserted into the intestinal wall allowing for the oral delivery of proteins and peptides which would otherwise be degraded in the intestinal tract.
Owner:RANI THERAPEUTICS
Pharmaceutical compositions and methods for fabrication of solid masses comprising Anti-interleukin antibodies
ActiveUS20150329633A1Minimize adverse effectsReduce volatilityPeptide/protein ingredientsMetabolism disorderIntestinal wallsAntiendomysial antibodies
Embodiments of the invention provide shaped masses comprising one or more drugs such as proteins or polypeptides and methods for forming such shaped masses. One embodiment provides a shaped mass comprising a drug such as a protein or polypeptide having a biological activity in the body of a mammal. The shaped mass is formed by compression of a precursor material comprising the drug wherein an amount of biologically active drug in the mass is a preserved above a minimum level. Drugs which may be incorporated into the shaped mass may include one or more glucose regulating proteins such as insulin, incretins; and immunoglobulins such as TNF-inhibiting antibodies or interleukin neutralizing antibodies. Embodiments of the shaped mass may be incorporated into a tissue penetrating member which is inserted into the intestinal wall allowing for the oral delivery of proteins and peptides which would otherwise be degraded in the intestinal tract.
Owner:RANI THERAPEUTICS
Pharmaceutical compositions and methods for fabrication of solid masses comprising tnf-inhibiting antibodies
ActiveUS20150329631A1Minimize adverse effectsReduce volatilityPeptide/protein ingredientsMetabolism disorderIntestinal wallsWhite blood cell
Embodiments of the invention provide shaped masses comprising one or more drugs such as proteins or polypeptides and methods for forming such shaped masses. One embodiment provides a shaped mass comprising a drug such as a protein or polypeptide having a biological activity in the body of a mammal. The shaped mass is formed by compression of a precursor material comprising the drug wherein an amount of biologically active drug in the mass is a preserved above a minimum level. Drugs which may be incorporated into the shaped mass may include one or more glucose regulating proteins such as insulin, incretins; and immunoglobulins such as TNF-inhibiting antibodies or interleukin neutralizing antibodies. Embodiments of the shaped mass may be incorporated into a tissue penetrating member which is inserted into the intestinal wall allowing for the oral delivery of proteins and peptides which would otherwise be degraded in the intestinal tract.
Owner:RANI THERAPEUTICS
Fillet freezing method
InactiveCN101919433ASolve the rapid freezing problemImprove water holding capacityMeat/fish preservation by freezing/coolingSodium lactateQuick Freeze
The invention discloses a frozen fillet processing method, belonging to the fields of frozen aquatic products and aquatic product processing, which can be popularized and applied to the freezing process of other foods. In the method of the invention, protective agent is added into boned skinless fillets by injecting, ultrasonic immersing or a combined manner of injecting and ultrasonic immersing for processing the fillets; the fillet quick freezing is realized by adopting the synergistic effect of indirectly contacted alcohol freezing solution immersing and ultrasound freezing; and the fillets are frozen and stored at below 18 DEG C. The protective agent is an aqueous solution containing sodium lactate, trehalose, unfrozen protein, Nisin and salt; the protein denaturation of the frozen fillets processed by the protective agent is reduced, the retention performance is improved, the nutritional value, flavor and mouthfeel can be maintained to the largest extent, the freezing time is short, and the production efficiency is high.
Owner:TIANJIN UNIV OF SCI & TECH
Nutritional composition and methods of making and using same
InactiveUS20060239987A1Treatment and/or prophylaxis of conditionsQuality improvementCosmetic preparationsOrganic active ingredientsStress inducedMedicine
A composition and method for treatment and / or prophylaxis of stressed-induced conditions and a method of forming the composition are disclosed. The composition includes hyaluronic acid and superoxide dismutase configured to mitigate denaturing of the superoxide dismutase in a digestive track of an animal. Mitigating the denaturing of the superoxide dismutase allows simultaneous administration of both the hyaluronic acid and superoxide dismutase to the circulatory and lymph systems of an animal.
Owner:MOLECULAR REGENIX
Method for preparing yogurt powder and yogurt ice cream powder by using yogurt powder
ActiveCN101617717AReduce denaturationImprove stabilityMilk preparationFrozen sweetsNon dairyChemistry
A method for preparing yogurt powder comprises the following steps: (1) deploying milk; (2) emulsifying and homogenizing; (3) sterilizing; (4) fermenting: adding 0.01-0.3 wt percent of direct vat ferments in the milk to form fermentation liquid after fermentation; (5) maturing and deploying: deploying the fermentation liquid with the following components in percentage by weight to form deployment solution, 66.1-92.3 fermentation liquid, 3.7-23.1 maltodextrin, 0-2.9 Beta-cyclodextrin, 0.15-6.4 non-dairy creamer, 0.36-2.45 stabilizing agents, 0.72-3.3 emulsifying agents and 0.36-2.45 lactic acid powder; (6) maturing: forming power spraying liquid; and (7) powder spraying: spraying and drying the power spraying liquid to form the yoghourt powder. The stabilizing agents are added in the deployment liquid before the powder spraying in the invention to package protein during the powder spraying, reduce the denaturalization of the protein and increase the later dissolving stability. The yogurt powder has natural fermented milk scent and can effectively promote the growth of intestinal bacteria.
Owner:SYNBIOTECH BIOTECHNOLOGY YANGZHOU CO LTD
Vehicle antifreeze
InactiveCN101619207AReduce denaturationReduce corrosionHeat-exchange elementsBoiling pointAntifreeze
The invention relates to vehicle antifreeze which comprises the following components in percentage by weight: 30-70 percent of ultrapure water, 18-50 percent of terylene grade glycol, 10-20 percent of industrial glycerin and 2-5 percent of accessories. Because the industrial glycerin is added in the formula of the antifreeze, the rubber denaturation of warm air pipes and the like is reduced, and the service life is prolonged. The addition of the industrial glycerin can reduce corrosions to metals, thereby enhancing the corrosion resistance and improving the boiling point. The antifreeze can be prepared at any freezing point within the range of between minus 15 DEG C and minus 60 DEG C and meet various requirements.
Owner:唐山三友矿山有限公司
Processing method of sweet potato sheet jelly
A processing method of sweet potato sheet jelly, comprising the following steps of cleaning raw material sweet potatoes by water, and then continuously conveying the sweet potatoes to a crusher for crushing into sweet potato slurry with the particle size smaller than 3mm, weighing the sweet potato slurry, sweet potato graham flour or / and sweet potato starch in a weight ratio of the sweet potato slurry, the sweet potato graham flour or / and the sweet potato starch, which is 1: 0-100, adding water, mixing uniformly to prepare mixed slurry, wherein the water is added so that the water content in the mixed slurry is 48-65% by weight percentage, sending the mixed slurry to a spiral extrusion molding machine for curing and molding to form the sheet jelly, conveying the sheet jelly by a continuous conveyer, automatically cutting the sheet jelly according to a fixed length, and packing through an automatic packing machine or a vacuum packing machine so as to prepare the sheet jelly product. The invention uses mechanical continuous production and has a simple processing technology. The prepared sweet potato sheet jelly product maintains the nutrient components in the sweet potatoes and the active components capable of improving human immunity, and is excellent green health food.
Owner:SICHUAN GUANGYOU SWEET POTATO & FOOD PROD CO LTD
Method of producing polymers of spider silk proteins
ActiveUS20120041177A1Easy to operateReadily be manipulated to polymerise into fibersPeptide/protein ingredientsPeptide preparation methodsFiberLiquid medium
A method of producing polymers of an isolated spider silk protein involves providing a solution of said spider silk protein in a liquid medium at pH 6.4 or higher and / or an ion composition that prevents polymerisation of the spider silk protein. The properties of the liquid medium are adjusted to a pH of 6.3 or lower and an ion composition that allows polymerisation of the spider silk protein. The spider silk protein is allowed to form polymers in the liquid medium, and the resulting spider silk protein polymers are isolated from the liquid medium. The resulting polymers are useful as fibers, films, foams, nets or meshes.
Owner:SPIBER TECH
Swelling pre-squeezing leaching preparation method for high oil-containing material
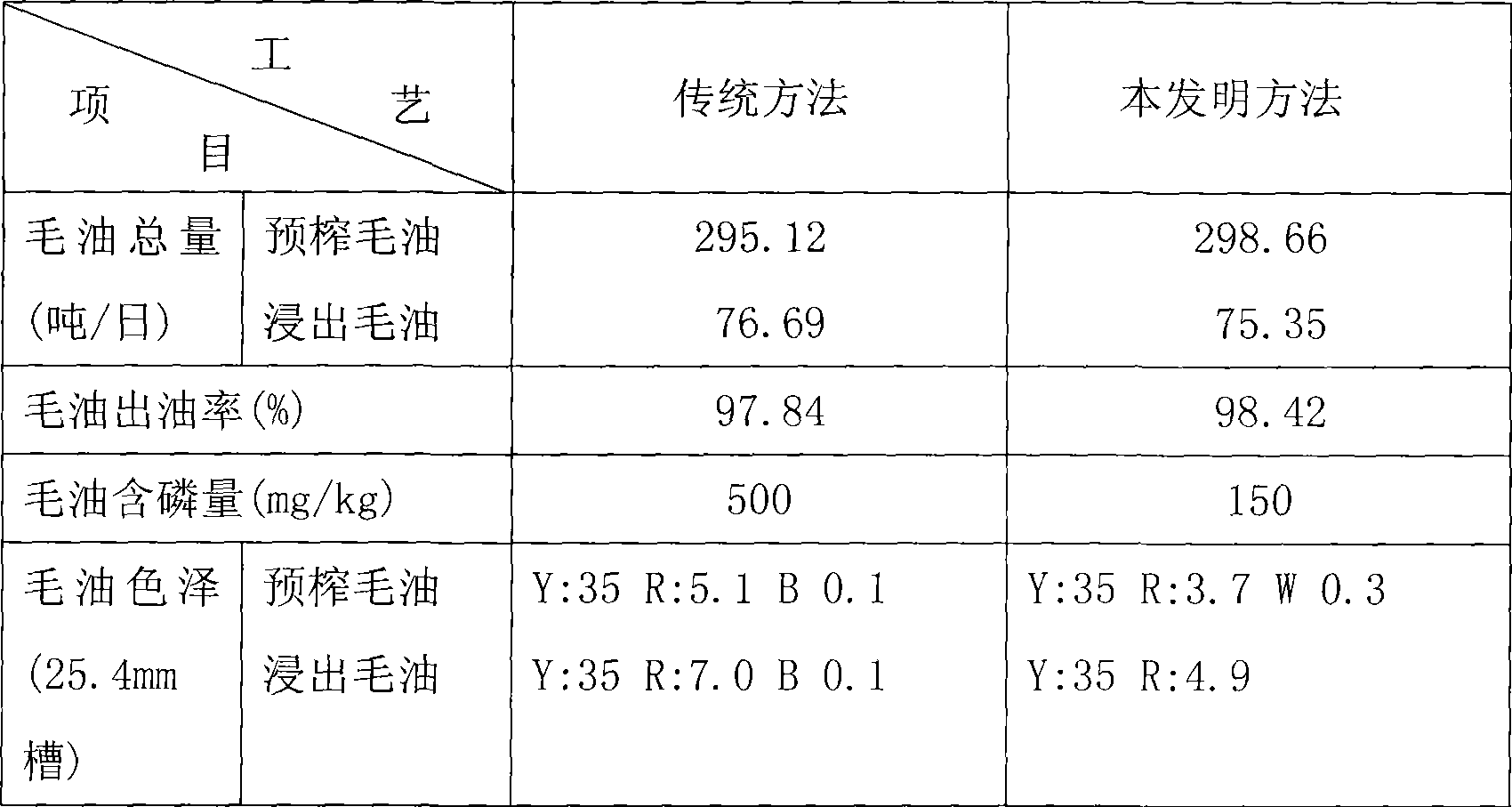
InactiveCN101235340AAvoid excessive denaturationReduces loss of lysineFatty-oils/fats productionWater contentOil content
The invention relates to a high oil-content oil material expanding prepressing leaching oil preparation method, which is characterized in that the method comprises the following steps, firstly, cleaning, cleaning oiling to remove impurities, secondly, tempering, adjusting the water content of oiling whose impurities are removed, thirdly, rolling slab, rolling oiling whose water content is adjusted into material slab, fourthly, squeezing and swelling, squeezing and swelling, the material slab, fifthly, drying, drying the material slab which is squeezed and swelled, sixthly, prepressing, prepressing the material slab which is dried to press one portion of oil, seventhly, leaching, leaching prepressed cake of the oil which is prepressed to get another portion of oil and meal from solvent extraction. A squeezing and swelling step in the invention is utilized to replace a cooking step in the existing method, which improves the quality and amounts of prepressing crude palm oil, increases handling capacity of a prepress expeller, and reduces power consumption and abrasion of a prepress expeller. The method of the invention is mainly applicable for oil preparation enterprises which utilize high oil-content oiling such as rapeseed and the like to be raw material.
Owner:中机康元粮油装备(北京)有限公司 +1
Medicine for treating chronic hepatitis b
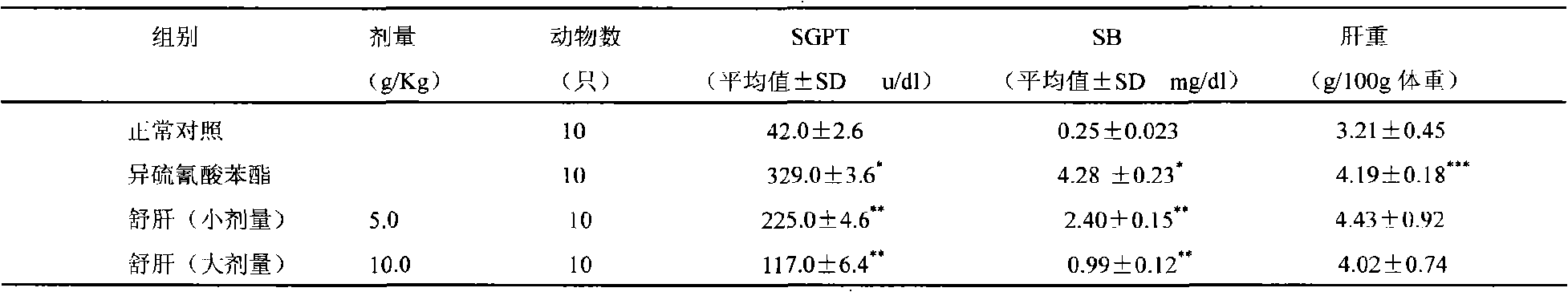
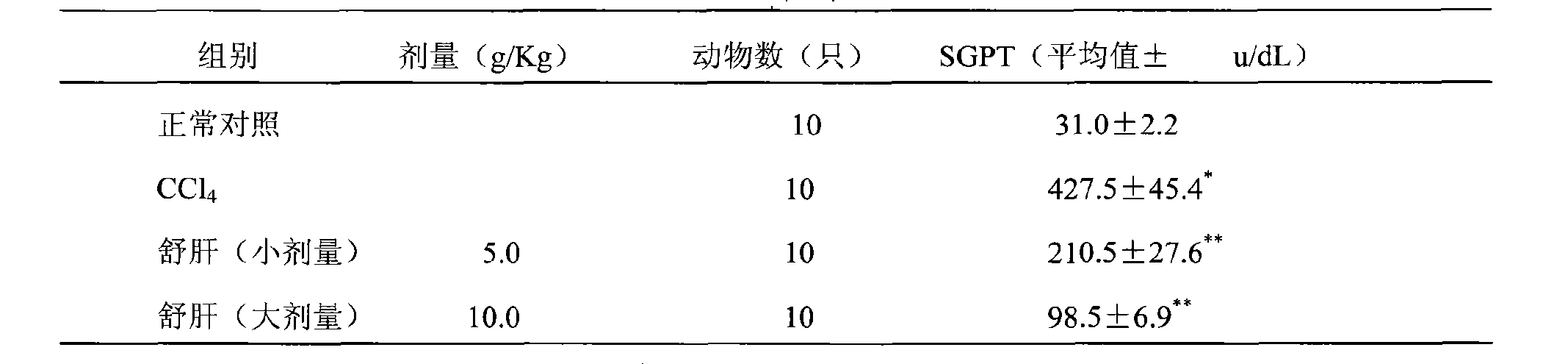
ActiveCN101357219AGood treatment effectGood curative effectDigestive systemAntiviralsSalvia miltiorrhizaChronic hepatitis
The invention discloses a medicine for treating chronic hepatitis B, which contains the raw materials by the mixture ratio: 6-18% of herba artemisiae capillaries, 1-7% of Chinese thorowax, 1-7% of angelica, 3-12% of radix paeoniae alba, 3-12% of salvia miltiorrhiza, 1-10% of radix curcumae, 1-7% of rhizoma corydalis, 1-7% of common burreed rhizome, 1-7% of rhizoma zedoariae, 1-7% of nutgrass galingale rhizome, 1-7% of fructus meliae toosendan, 1-10% of dangshen, 1-7% of largehead atractylodes rhizome, 6-18% of radix astragali, 3-12% of indian bread, 1-7% of immature bitter orange, 1-7% of amomum villosum, 1-7% of areca, 0.5-5% of Chinese eaglewood and 1-7% of liquorice; the medicine is prepared by adopting conventional method. The medicine has the efficacies of clearing heat and promoting diuresis, nourishing the liver and invigorating the spleen, regulating the flow of qi and removing blood stasis, and can be used for treating chronic hepatitis B with remarkable effects, short period of treatment and rapid effects.
Owner:LANZHOU FOCI PHARM CO LTD
Automatic acid mixing apparatus and method thereof, and automatic pipeline iodic acid apparatus and method thereof
ActiveCN102351945AUniform precipitationRapid precipitationPeptide preparation methodsOther dairy technologyHigh concentrationAutomatic control
An objective of the invention is to provide an automatic acid mixing apparatus and a method thereof, and an automatic pipeline iodic acid apparatus and a method thereof. Therefore, problems of nonuniform mixing and over high local acidity are solved, wherein the problems exist in a current on-line souring system. The automatic pipeline iodic acid apparatus comprises a material pipeline, a material variable frequency pump, an acid pipeline, a diluted acid variable frequency pump, a Venturi tube and a mixer; an entrance section of the Venturi tube is connected with a premixer; an acid jetting pipeline is arranged in the premixer; and a tail portion of the mixer is connected with an iodic acid pH monitor, which is connected with an automatic control module. according to the invention, the automatic pipeline iodic acid apparatus is utilized to enable automatic iodic acid to be realized; a diluted acid that is configured by the automatic iodic acid apparatus enters the automatic acid mixing apparatus to carry out automatic acid mixing. Besides, materials can be mixed fully, rapidly and high efficiently; occurrences of unstable acid concentration and a local peracid phenomenon during the iodic acid processing can be effectively avoided; and denaturation of active materials with acid nonresistant performance on the condition of acid with high concentration can be reduced.
Owner:甘肃华羚乳品股份有限公司
Spider Silk Proteins and Methods for Producing Spider Silk Proteins
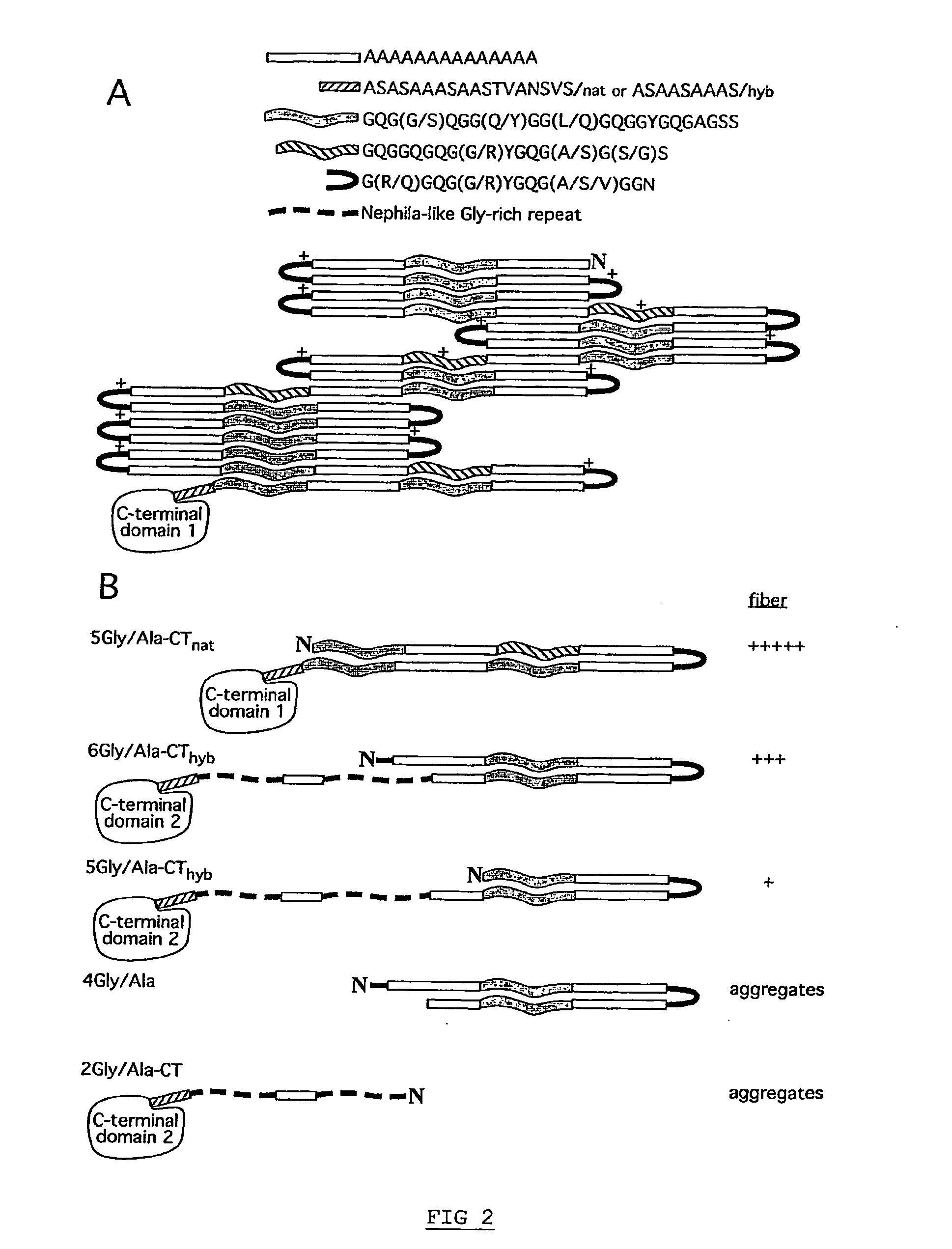
ActiveUS20090226969A1Easy to operateReduce denaturationMonocomponent protein artificial filamentSugar derivativesSpider ProteinsProtein Fragment
The invention provides an isolated major ampullate spidroin protein, which consists of from 150 to 420 amino acid residues and is defined by the formula KEP-CT. KEP is a repetitive, N-terminally derived protein fragment having from 80 to 300 amino acid residues. CT is a C-terminally derived protein fragment having from 70 to 120 amino acid residues. The invention further provides an isolated fusion protein consisting of a first protein fragment, which is a major ampullate spidroin protein, and a second protein fragment comprising a fusion partner and a cleavage agent recognition site. The first protein fragment is coupled via said cleavage agent recognition site to the fusion partner. The invention also provides a method of producing a major ampullate spidroin protein and polymers thereof.
Owner:SPIBER TECH
Single screw rod oilseed cold pressing expeller
The invention relates to a single-screw oil material cold squeezer which comprises a squeezing cage and shaft component, which consists of a squeezing cage part and squeezing shafts; and the squeezing shaft is positioned in the squeezing cage part. The cold squeezer is characterized in that the squeezing shaft comprises a squeezing screw mainshaft, a squeezing screw, a cake forming ring and a cake pulverization ring; and the squeezing screw, the caking forming ring and the cake pulverization ring are sleeved and fixed on the outside of the squeezing screw mainshaft. Spiral of the squeezing screw is broken. The squeezing cage part comprises a squeezing cage and a scraper; the scraper is arranged on the squeezing cage; and the scraper is centripetally inserted into a disconnection part of the spiral of the squeezing screw. The squeezing shaft comprises a first squeezing section, a second squeezing section and a third squeezing section. Under the condition of low temperature, the cold squeezer can squeeze an oil material, squeezes out most lipin of the oil material, ensures low denaturing performance of protein in the oil material, is suitable for single squeezing process and is also suitable for presqueezing and leaching process.
Owner:中机康元粮油装备(北京)有限公司 +2
Preparation of lotus seed protein polypeptide
The invention discloses a preparation method of lotus seed protein polypeptide. Lotus seed protein is extracted by using a low-concentration weak base Ca (OH)2 to replace a strong base NaOH, so as to greatly reduce the influence of the strong base to raw materials and reduce the denaturalization of protein glomeration and residual lotus seed starch during the extracting process; the extracted protein has good color and taste; meanwhile, lotus nut amylum can be taken as the raw materials of other lotus seed foods additionally. In addition, low-concentration of Ca ion is more advantageous to human beings to absorb the protein, can also make up calcium deficiency and is not required to be removed; and desalting step is not needed during the extracting process of protein. In addition, the pH is not required to be adjusted during the hydrolytic process of acid protease; the hydrolysis process is simple, and the callback is not required simultaneously, the desalting process is eliminated, so that the cost is saved greatly. The hydrolysis of the acid protease is proper, so that the bitterness of products caused by the generation of a large amount of small peptide after excessive hydrolysis is avoided. The lotus seed protein polypeptide has the yield of 71.4 percent and good color and taste.
Owner:XIANGTAN UNIV
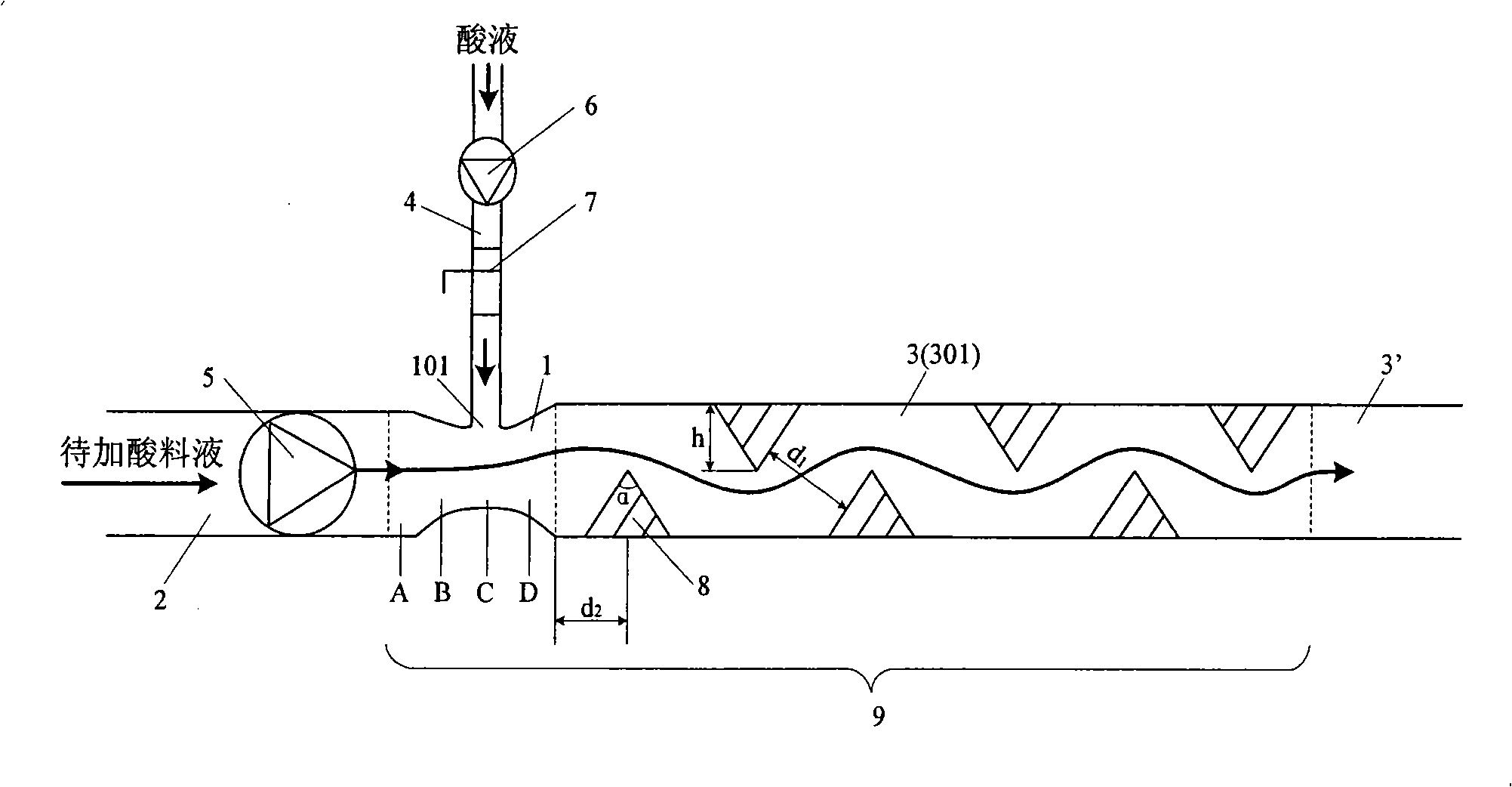
Online acid addition method
The invention provides an online acid adding method which adds acid liquid to a material liquid to be added with acid by adopting a Venturi tube to form an acidic material liquid. The Venturi tube includes an entrance segment, a contraction segment, a throat segment and a diffusion segment, wherein the entrance segment is connected with the pipe of the material liquid to be added with acid; the diffusion segment is connected with a static mixer; and an acid liquid inlet is arranged on the throat segment of the Venturi tube. The online acid adding method comprises the following steps: introducing the material liquid to be added with acid into the Venturi tube from the entrance segment, and flowing to contraction segment, the throat segment and the diffusion segment; and introducing the acid liquid from the throat segment of the Venturi tube into the Venturi tube, online mixing with the material liquid flowing to the diffusion segment to form the acidic material liquid, and flowing to the static mixer through the diffusion segment for further mixing. The online acid adding method can achieve good mixing effect through the dynamic quantitative mixing process of the acid liquid and the material liquid according to the ratio.
Owner:INNER MONGOLIA YILI INDUSTRIAL GROUP CO LTD
Stabilizing catheter for protein drug delivery
InactiveUS20020156434A1Reduce denaturationDiminishing unfoldingOther blood circulation devicesGlovesInsulin infusionINSULIN PREPARATIONS
Stabilizing catheters for delivery of one or more protein drugs to a patient. The stabilizing catheter embodiments of the invention maintain or preserve a biologically / pharmacologically active form of the protein drug for delivery to a site within the body. Particular embodiments include a tubing layered with a hydrophilic and mobile polymer that aids in the maintenance or preservation of an active conformer of the protein drug. These embodiments of the stabilizing catheter prevent site loss of protein drugs, such as insulin. Other embodiments include a tubing that is layered with a material that substantially prevents diffusion of small, insulin formulation-stabilizing molecules out from the catheter, as well as substantially prevents the diffusion of small, insulin formulation-destabilizing molecules into the catheter, during a period of insulin infusion. In effect, these embodiments of the stabilizing catheter maintain the stabilizing effect of a particular insulin formulation, and consequently, substantially prevents occlusions / deposits from being formed during a period set for insulin delivery. Still other embodiments are directed to a combination of these features of the stabilizing catheters of the invention.
Owner:MEDTRONIC MIMIMED INC
Soybean milk and dry-process preparation method thereof
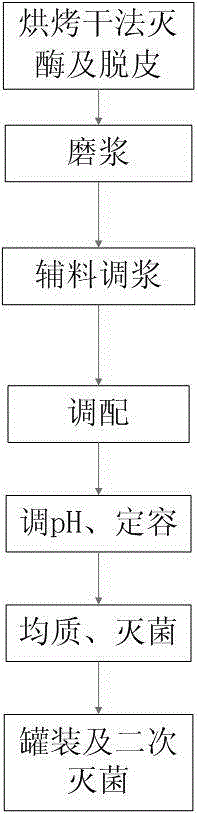
InactiveCN104814156AReduce the residue of soil bacteriaRemove beany smellMilk substitutesFood scienceProcess engineeringSoy milk
The invention discloses soybean milk. The soybean milk comprises the following components in parts by weight: 60-68 parts of soybeans, 50-65 parts of white sugar, 850-875 parts of water, 2-4 parts of a soybean-milk stabilizing agent and 0.8-1.5 parts of other auxiliary materials, wherein the pH is 7.0-7.3. A dry-process preparation method for the soybean milk comprises the following steps: (1) adopting a baking dry process for enzyme deactivation and peeling, namely sieving the soybeans to remove impurities, putting into a baking oven, baking for 10-18 minutes under the temperature of 100-110 DEG C, taking out the soybeans when the soybeans have the 40%-50% cooking degree, cooling and peeling; (2) grinding into soybean milk, namely carrying out coarse crushing on the peeled soybeans in the step (1) till irregular particles with the particle diameter being 2-3mm appear, then adding water with the weight being 2-2.5 times of the weight of the soybeans, and grinding the soybeans in the form of irregular particles into the soybean milk; (3) adding the auxiliary materials for mixing the soybean milk: (4) blending; (5) adjusting the pH, and adding water to obtain the constant volume; (6) homogenizing and sterilizing; and (7) carrying out canning and secondary sterilization. The dry-process preparation method has the advantages that the production period is short, the cost is low and the production operation is easy.
Owner:湖南湘鹰食品科技有限公司
Optically active butylphthalide open-ring derivative, preparation method and medical application
InactiveCN103193789AFuzzy structureReduce the numberNervous disorderOrganic chemistryDiseaseAntithrombotic Agent
The present invention relates to the field of pharmaceutical chemistry and therapeutics, and particularly relates to an optically active butylphthalide open-ring derivative as shown in the general formula I or a pharmaceutically acceptable salt thereof and a pharmaceutically acceptable carrier, preparation methods thereof, pharmaceutical compositions containing the compounds, and medical application thereof, especially application in medicines for prevention and treatment of cardiovascular and cerebrovascular and improvement of heart and brain circulatory disturbance, antiplatelet aggregation medicines, antithrombotic medicines, anti-ischemic medicines, anti-dementia medicines, anti-atherosclerotic medicines, and medicines for anti-diabetes and complications thereof. Pharmacological experimental results show that the compounds have good anti-platelet aggregation activity, anti-thrombotic activity, anti-ischemic activity and neuroprotective effects, and are clinically useful for the preparation of medicines for preventing or treating diseases associated with platelet aggregation.
Owner:CHINA PHARM UNIV
Method for preparing high-strength collagen tissue repair material product
InactiveCN101564552AIncrease crosslink densityHigh strengthCatheterProsthesisTissue repairVolumetric Mass Density
The invention relates to a method for preparing a high-strength collagen tissue repair material product. The method obtains the final collagen product through the treatment steps of salting out, centrifugation, dehydration, crosslinking and drying of a collagen solution. Because the treatment method adopts the mode of improving the solid content of collagen in collagen gel and the crosslinking density in the collagen gel, the mechanical property of the final product is greatly improved. Simultaneously, because the adopted method does not influence the biological property of the collagen, the final collagen product still maintains good biological activity. The collagen product can be used for the repair treatment of articular cartilage, meniscus and skin, the construction of artificial blood vessels and nerve conduits, and the like.
Owner:SOUTH CHINA UNIV OF TECH
Regulation and control method of mechanical strength of natural biologic material products
InactiveCN101648035AGood biological activityReduce denaturationProsthesisPorosityVolumetric Mass Density
The invention discloses a regulation and control method of mechanical strength of natural biologic material products. A final product is obtained by the treating steps of freezing and drying, compression, crosslinking and secondary drying on natural biologic material solution. The treating method adopts the controllable compression step for a primary product after freezing and drying, thereby controlling the density of a porous material product, reducing the porosity of the porous material product and controlling the mechanical performance of the final product by the step. Meanwhile, the adopted method does not influence the biologic performance of the initial natural biologic material, thereby still keeping better biologic activity of the final product.
Owner:SOUTH CHINA UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com