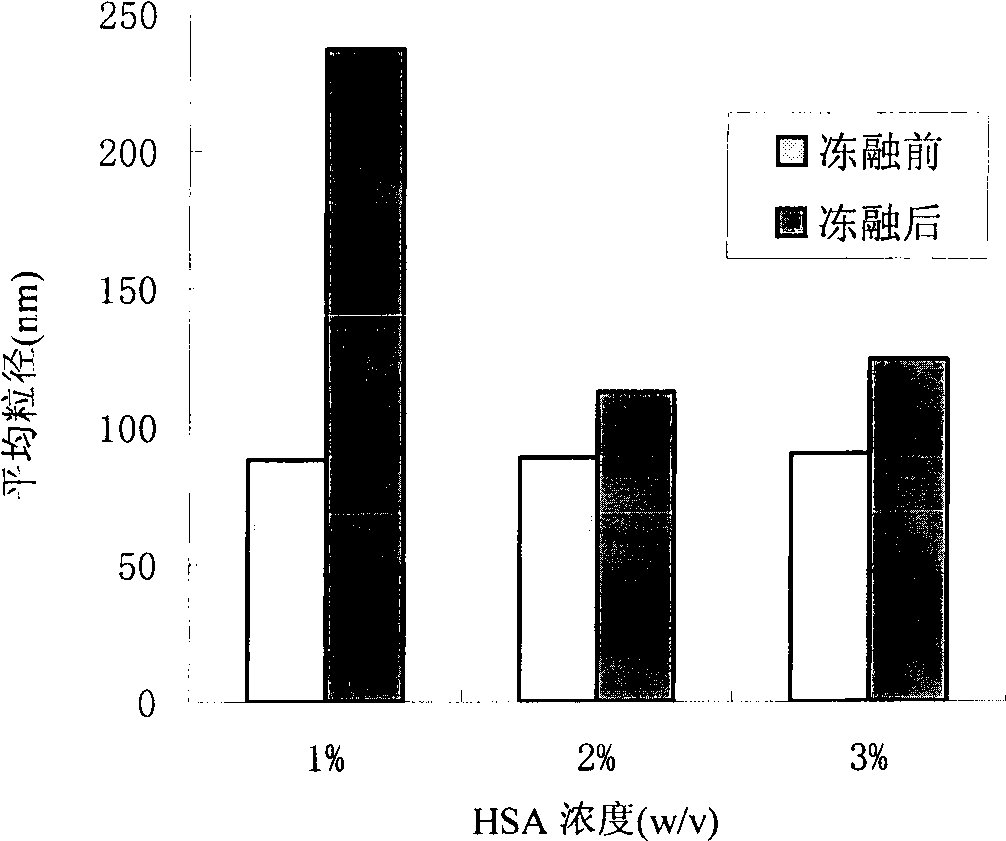
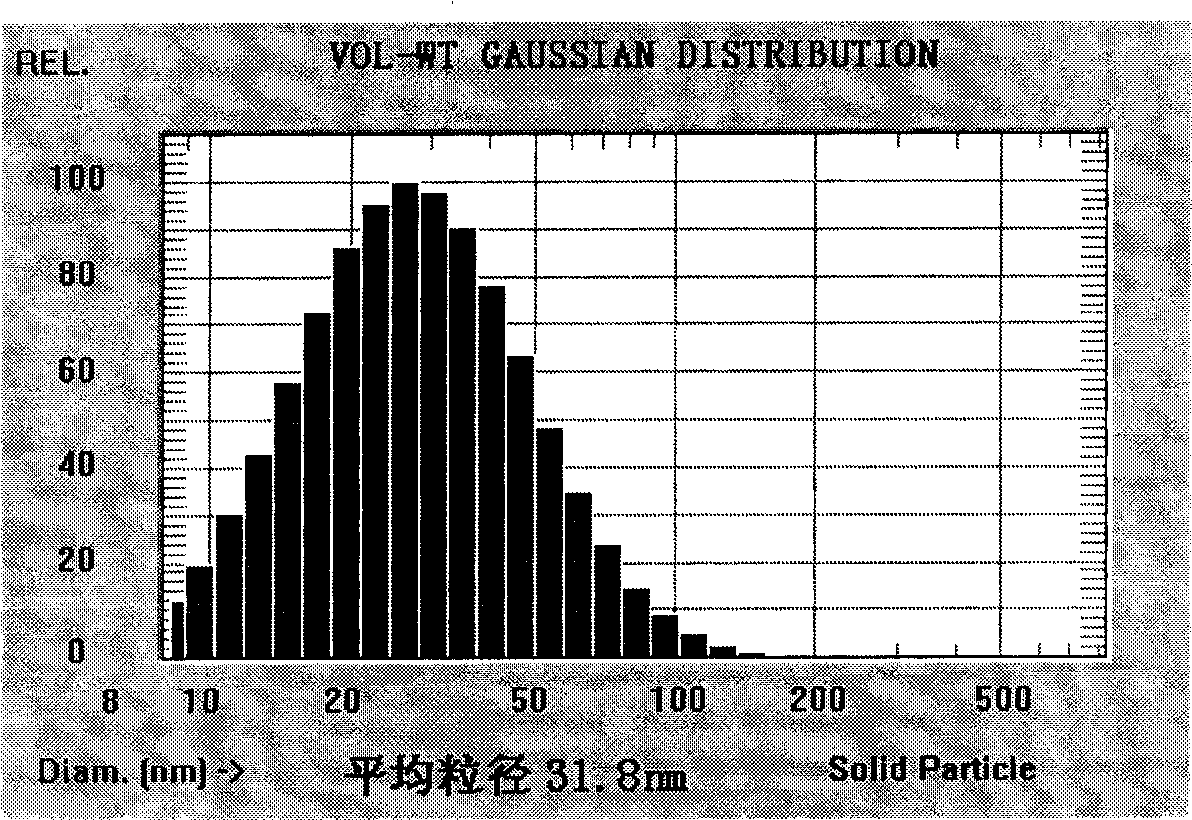
Drug delivery system and preparation method thereof
A drug delivery system and protein technology, which can be used in pharmaceutical formulations, medical formulations without active ingredients, and medical formulations containing active ingredients, etc. Can not identify caveolin and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Example 1 Problems in the preparation of cucurbitacin HSA nanoparticles by probe ultrasonic method
[0093] Cucurbitacin B 0.01g
[0094] HSA 4g
[0095] Appropriate amount of water
[0096] Dissolve cucurbitacin B (purity greater than 98%) in 1 ml of ethanol for later use; the prescribed amount of HSA is dissolved in water to prepare a 4% solution. The HSA solution was added to the cucurbitacin B ethanol solution, and after dispersion, it was placed in an ultrasonic instrument. After 200W ultrasonic treatment, and then 600W ultrasonic dispersion for 5min, the emulsion was obtained. The organic solvent was removed by rotary evaporation. The average particle diameter of the obtained nanoparticles is 282.2nm, the distribution is very wide, and it cannot pass through the microporous membrane of 0.45 μm, and precipitation will occur after standing for 20 minutes, and the system is unstable. After freeze-drying, the dispersibility is poor, and it is difficult to return ...
Embodiment 2
[0097] Example 2 Adding Phospholipids to Solve the Problems Existing in the Preparation of Cucurbitacin HSA Nanoparticles by Probe Ultrasonic Method
[0098] Cucurbitacin B 0.01g
[0099] HSA 4g
[0100] S100 1.5g
[0101] Appropriate amount of water
[0102] Cucurbitacin B (purity greater than 98%) and S100 (soybean lecithin, SPC Germany LIPOID) were dissolved in 1 ml of ethanol for later use; the prescribed amount of HSA was dissolved in water to prepare a 4% solution. Add the HSA solution to the cucurbitacin B / phospholipid ethanol solution, and place it in an ultrasonic apparatus after dispersion. After 200W ultrasonic treatment, and then 600W ultrasonic dispersion for 5min, the emulsion was obtained. The organic solvent was removed by rotary evaporation. The obtained nanoparticles have an average particle size of 156nm and a narrow distribution, and can pass through microporous membranes of 0.45 μm and 0.3 μm without precipitation after being placed for 60 minutes, an...
Embodiment 3
[0103] Example 3 Preparation of cucurbitacin HSA nanoparticles by dissolving phospholipids with tert-butanol
[0104]Cucurbitacin B 0.01g
[0105] HSA 4g
[0106] S100 1.5g
[0107] Appropriate amount of water
[0108] Cucurbitacin B (purity greater than 98%) and S100 (soybean lecithin, SPC Germany LIPOID) were dissolved in 5ml tert-butanol for later use; the prescribed amount of HSA was dissolved in water to prepare a 4% solution. Add the HSA solution into the cucurbitacin B / phospholipid tert-butanol solution, and place it in an ultrasonic apparatus after dispersion. After 200W ultrasonic treatment, and then 600W ultrasonic dispersion for 5min, the emulsion was obtained. The obtained nanoparticles have an average particle size of 139nm and a narrow distribution, and can pass through microporous membranes of 0.45 μm and 0.3 μm without precipitation after being placed for 60 minutes, and the system stability is greatly improved. After freeze-drying, the dispersibility is g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com