Method for preparing freeze-dried human blood coagulation factor VIII
A human coagulation factor and freeze-drying technology, which is applied in the preparation method of peptides, coagulation/fibrinolytic factors, factor VII, etc., can solve the problems of no medicine available, poor product appearance, severe opalescence, etc., and improve the safety of use Sexuality, avoiding denaturation and inactivation, and the effect of clarifying the reconstituted solution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
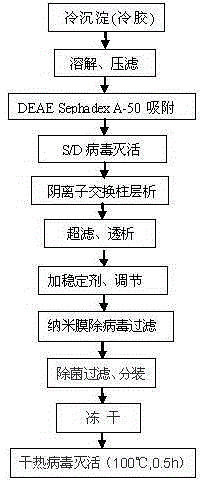
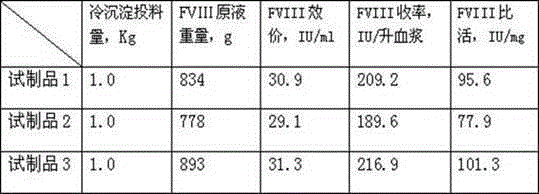
[0027] 1. Dissolving and filtering cryoprecipitate: put 1kg cryoprecipitate into 9kg dissolution buffer (0.02MTRIS-HCL, 0.15MNaCL, pH6.50-6.60), add heparin to the dissolution buffer to 2000IU / kg in advance, and control the temperature at 15 Stir at -20°C for 4 hours to make it fully dissolve, then filter with a Supradur50P filter plate produced by PALL in series with a 0.45 μm filter element, wash the filter element afterward, and finally collect 10.8 kg of clarified filtrate, pre-wash the filter plate and filter element;
[0028] 2. DEAEsephadexA-50 gel adsorption: Add 10.8g of DEAEsephadexA-50 gel to the above filtrate, and use hot water for injection above 70°C to swell and equilibrate the buffer before adding the gel (0.02MTRIS-HCL, 0.15MNaCL, PH6.50 -6.60) Pre-balanced, fully stirred for 1.5 hours after adding, then filtered with a filter cloth, collected the filtrate, and reused after the gel was regenerated;
[0029] 3. S / D virus inactivation: add Tween80 to 1.0% (wt%...
Embodiment 2
[0037] 1. Dissolving and filtering cryoprecipitate: put 1kg cryoprecipitate into 19kg dissolving buffer (0.02MTRIS-HcL, 0.075MNaCL, pH7.40-7.50), add heparin to the dissolving buffer in advance to 10000IU / kg, and control the temperature at 25 Stir at -30°C for 2 hours to make it fully dissolve, then filter with a Supradur50P filter plate produced by PALL in series with a 0.45 μm filter element, wash the filter element afterward, and finally collect 21.2 kg of clarified filtrate, pre-wash the filter plate and filter element;
[0038] 2. DEAESephadexA-50 gel adsorption: Add 10.6g of DEAESephadexA-50 gel to the above filtrate, and use hot water for injection above 70°C to swell and equilibrate the buffer before adding the gel (0.02MTRIS-HCL, 0.075MNaCL, PH7.40 -7.50) pre-balanced, fully stirred for 1 hour after adding, then filtered with a filter cloth, collected the filtrate, and reused after the gel was regenerated;
[0039] 3, S / D virus inactivation: with embodiment one;
[...
Embodiment 3
[0045] 1. Cryoprecipitate dissolution: put 1kg cryoprecipitate into 14kg dissolution buffer (0.02MTRIS-HcL, 0.1MNacL, pH6.90-7.10), add heparin to the dissolution buffer in advance to 6000IU / kg, and control the temperature at 20-25 ℃, stirring for 3 hours to make it fully dissolved; then filter with a Supradur50P filter plate produced by PALL in series with a 0.45 μm filter element, wash the filter element afterward, and finally collect 15.9 kg of clarified filtrate, and pre-wash the filter plate and filter element with dissolution buffer before filtration;
[0046] 2. DEAESephadexA-50 gel adsorption: Add 12g of DEAESephadexA-50 gel to the above filtrate, and use hot water for injection above 70°C to swell and equilibrate the buffer before adding the gel (0.02MTRIS-HCL, 0.1MNaCL, PH6.90- 7.10) Balance in advance, stir thoroughly for 45 minutes after adding, then filter with filter cloth, collect the filtrate, regenerate the gel and reuse it;
[0047] 3, S / D virus inactivation:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com