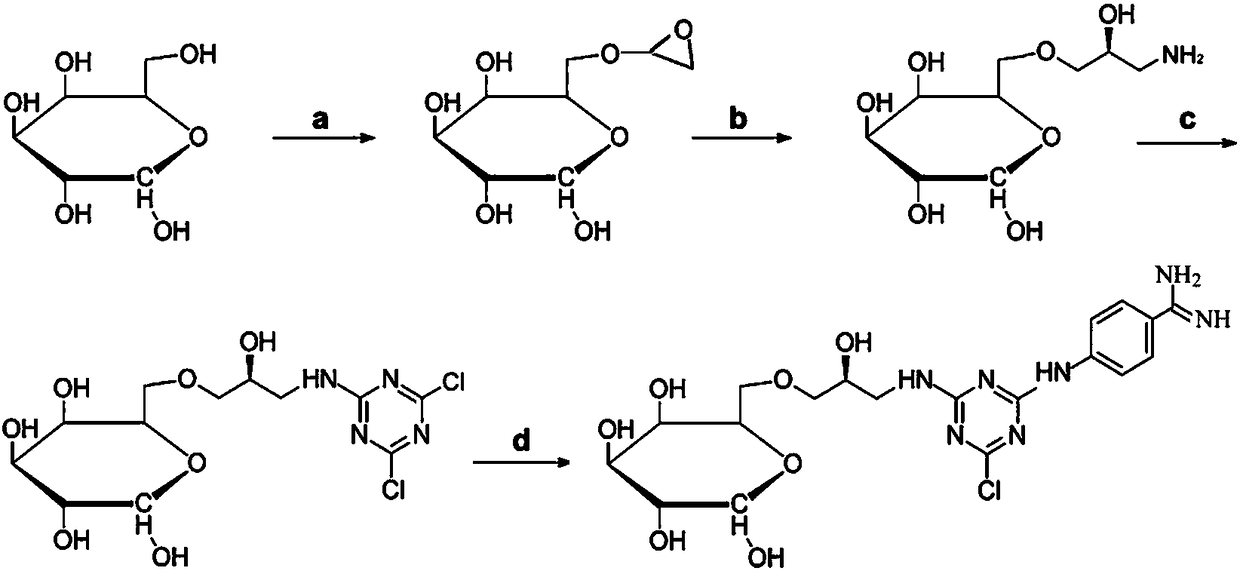
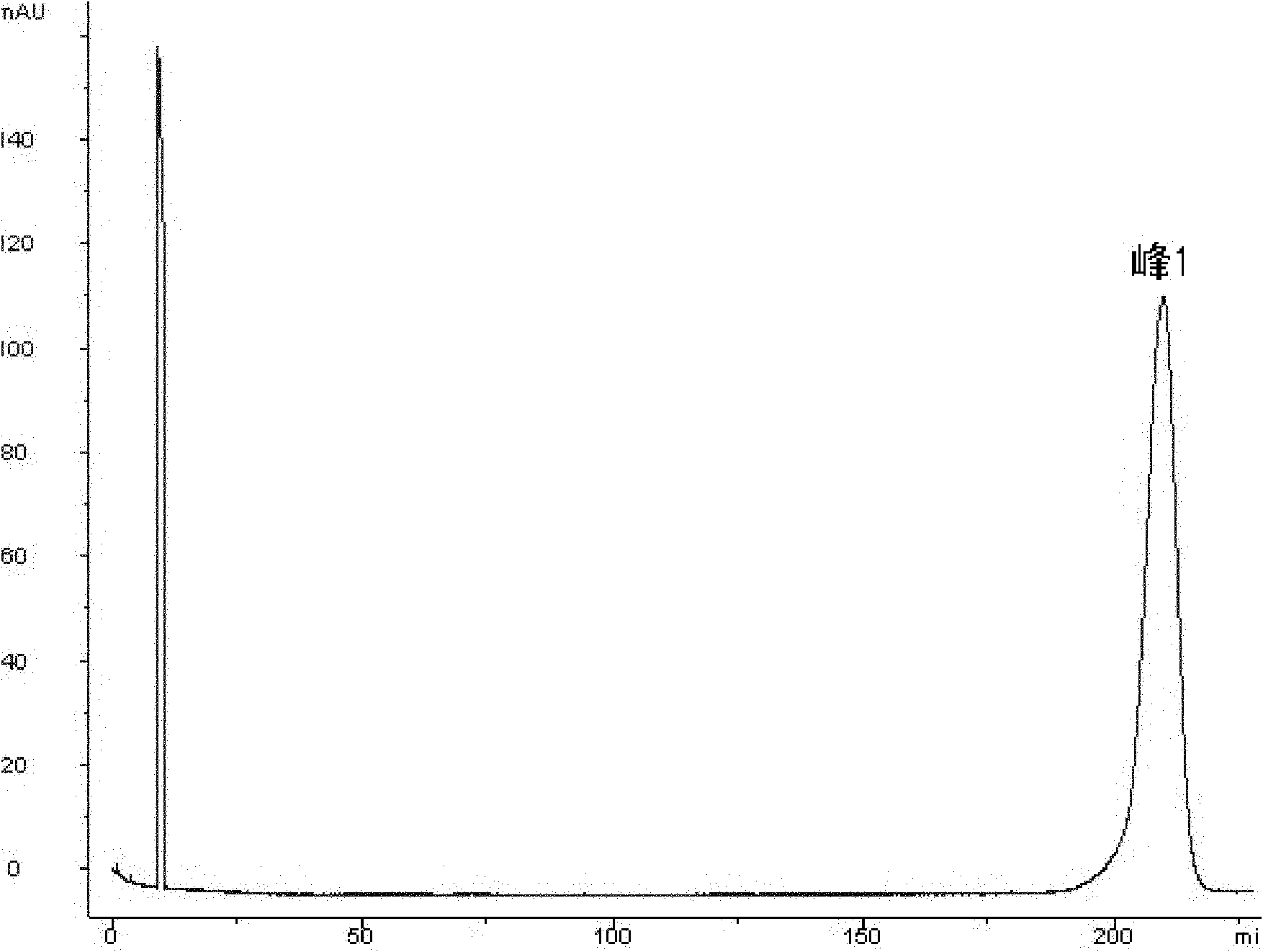
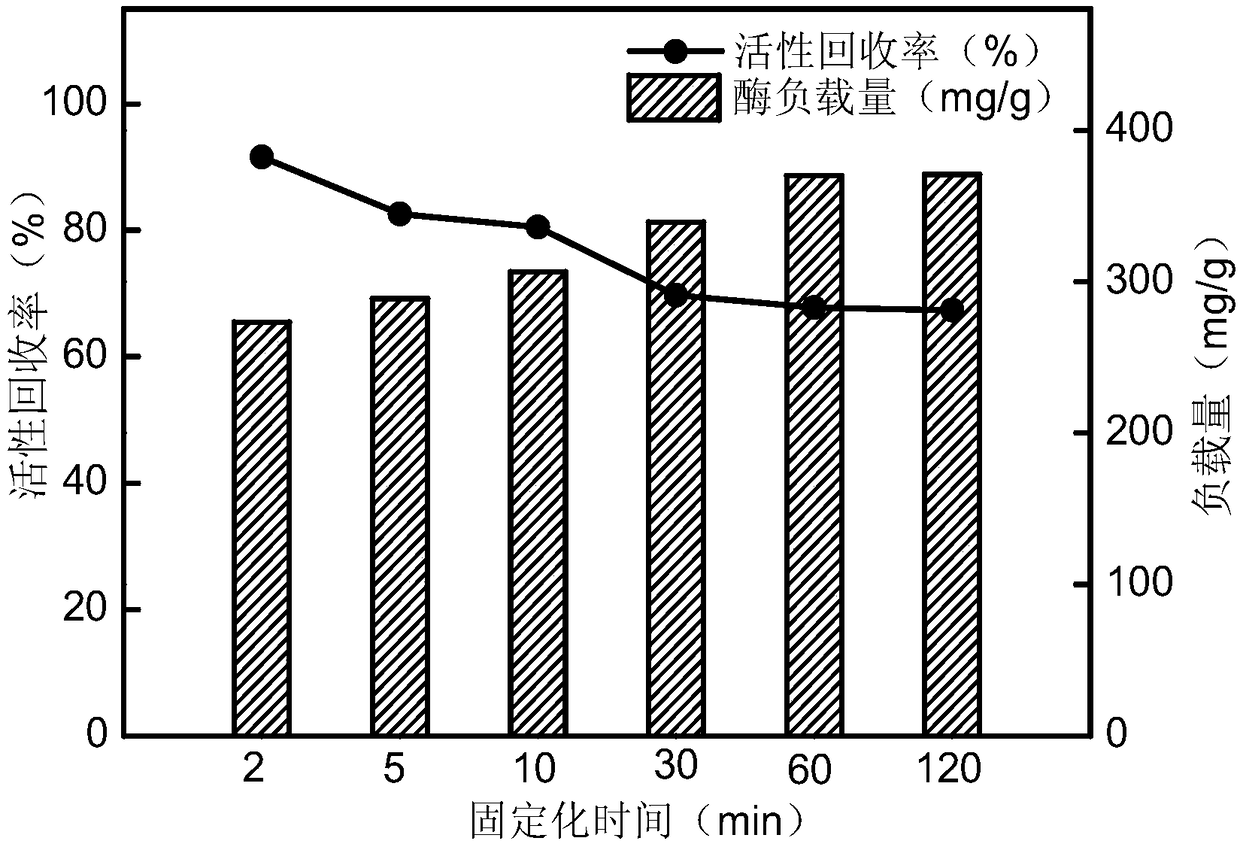
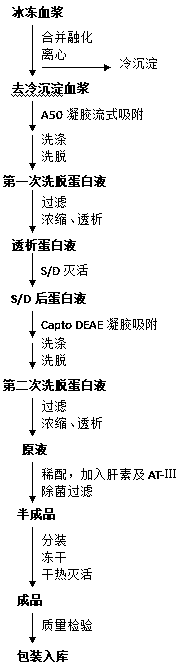
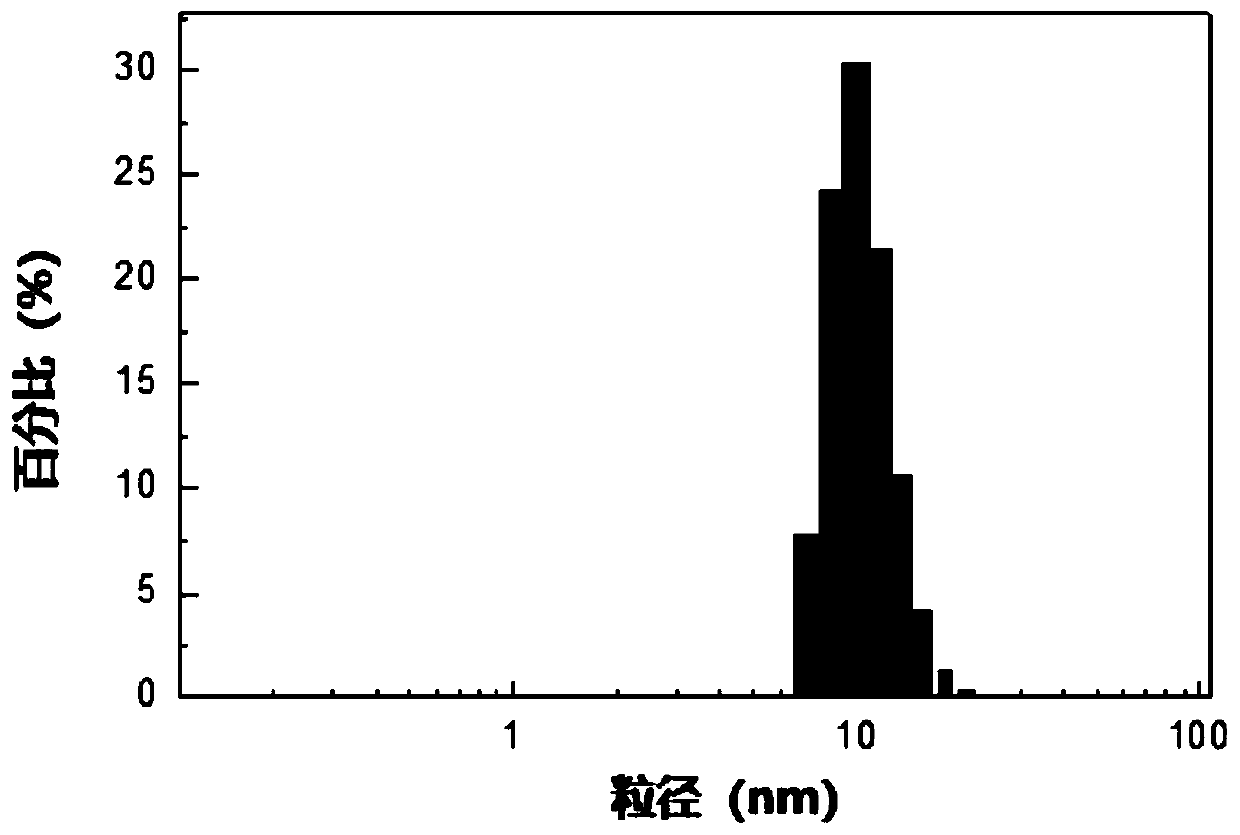
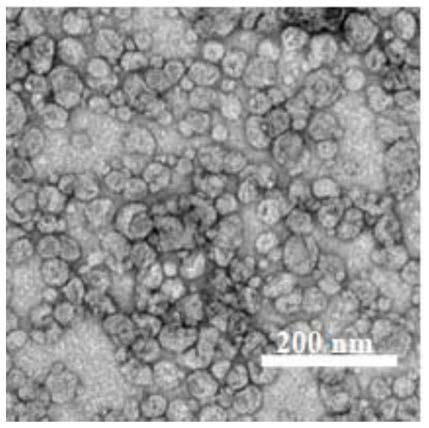
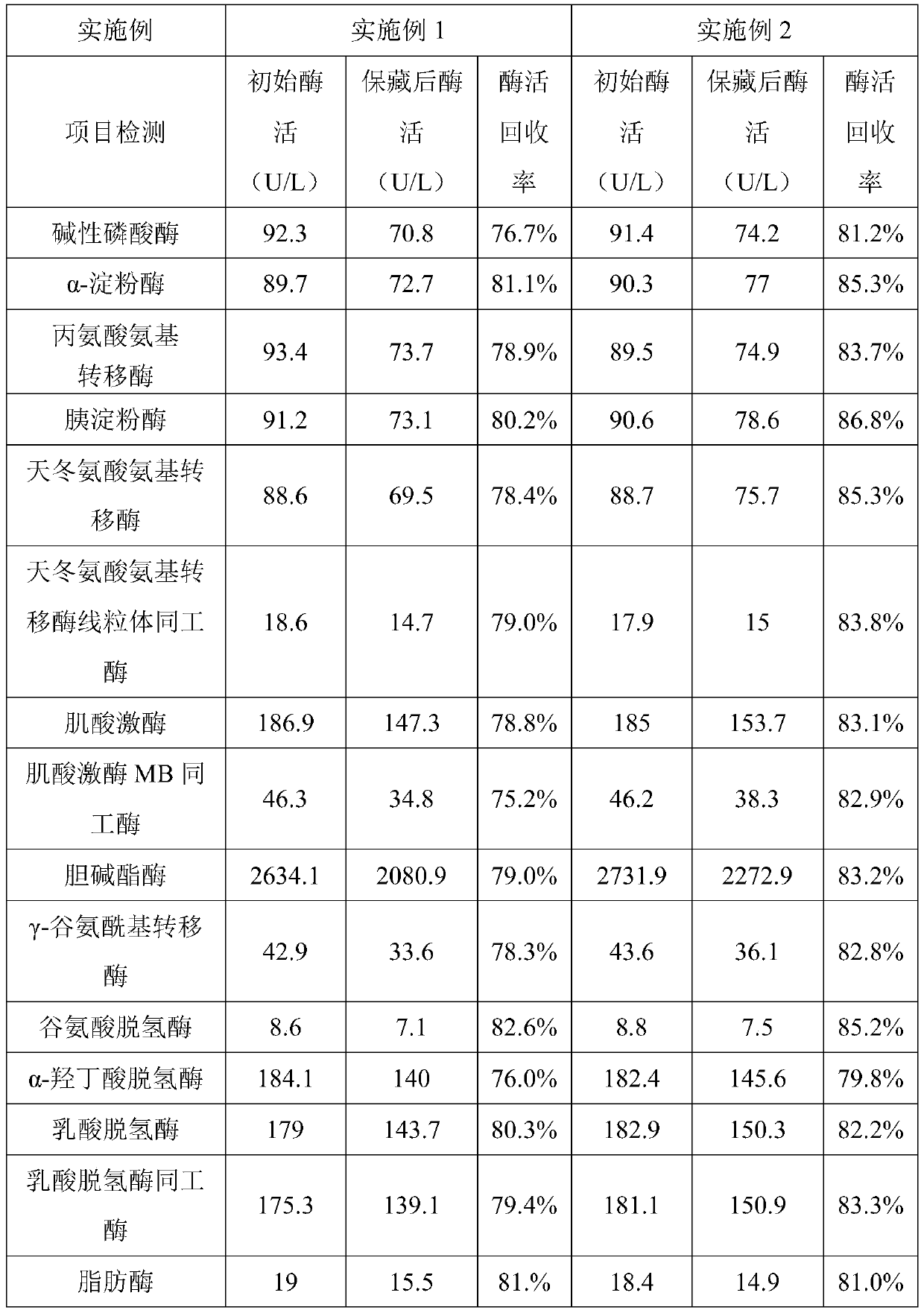
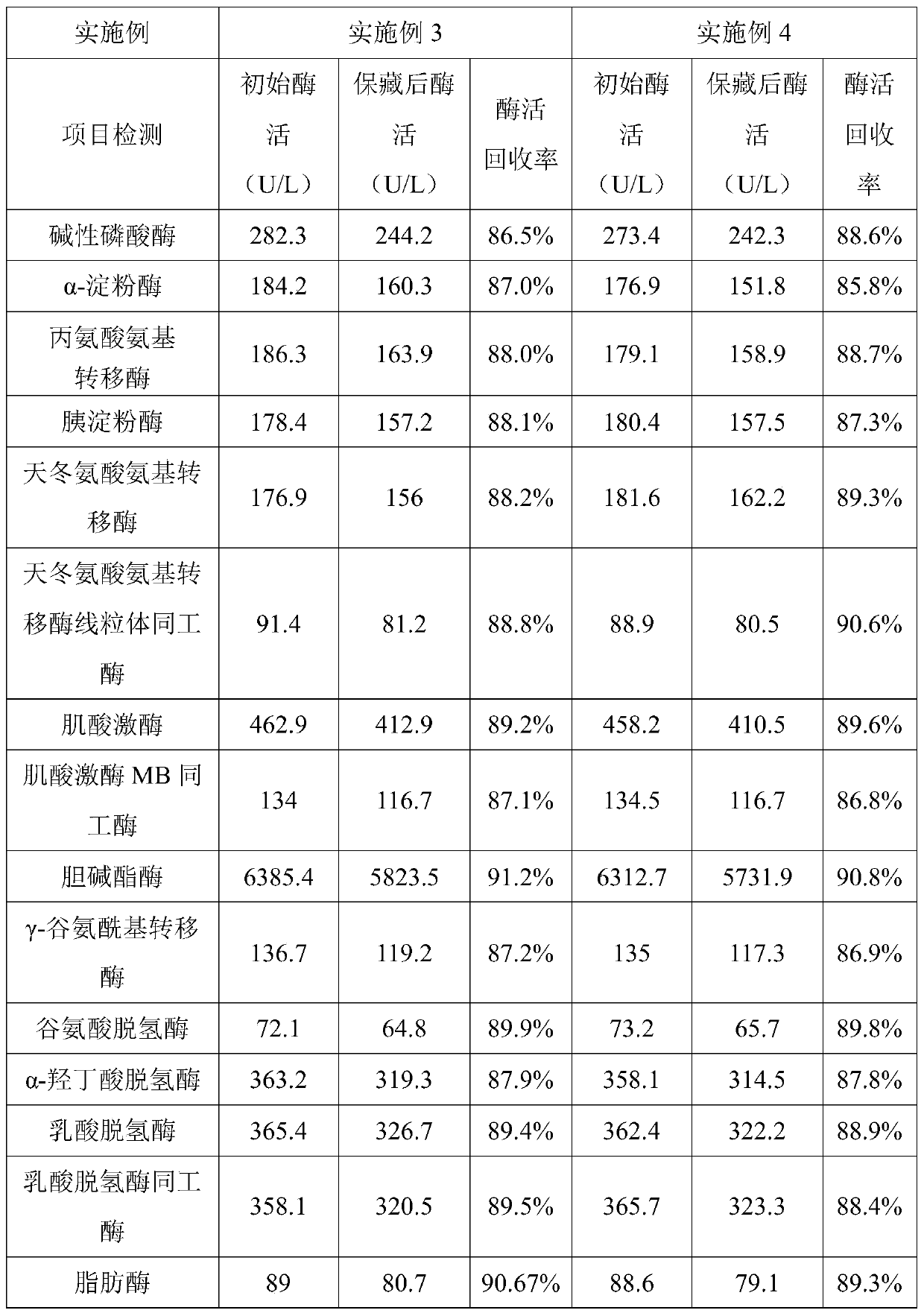
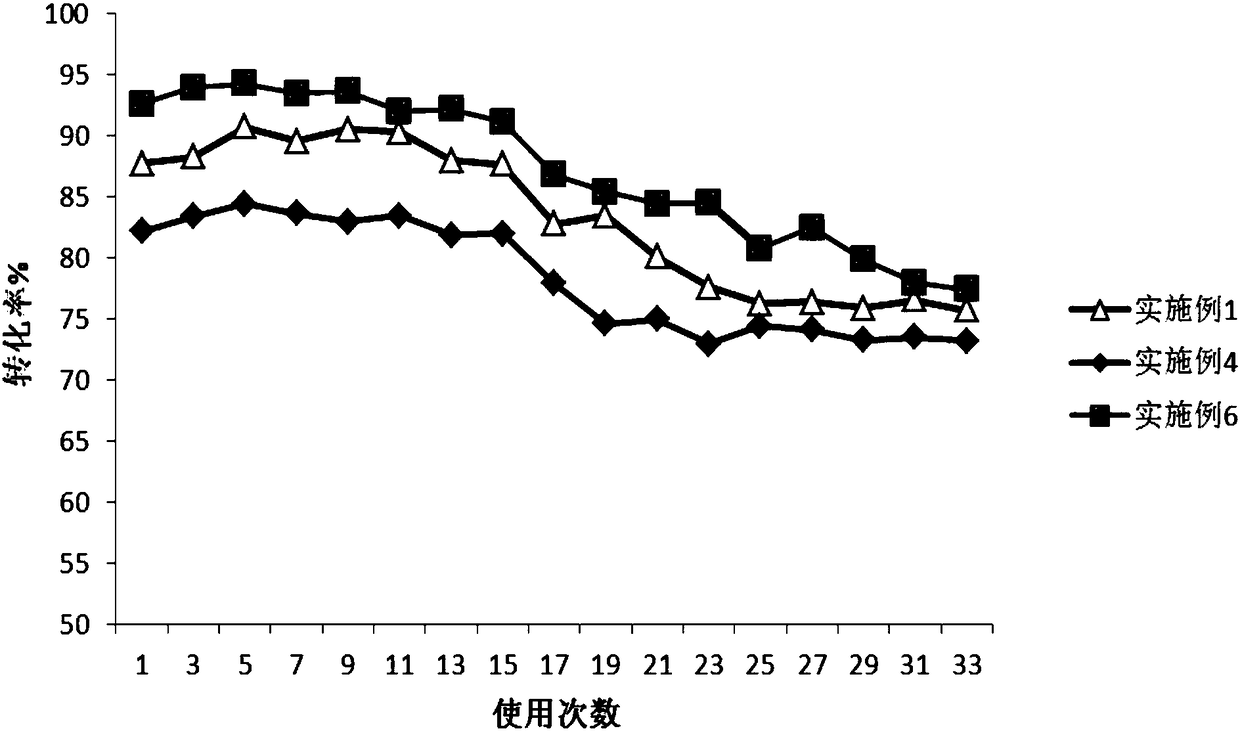
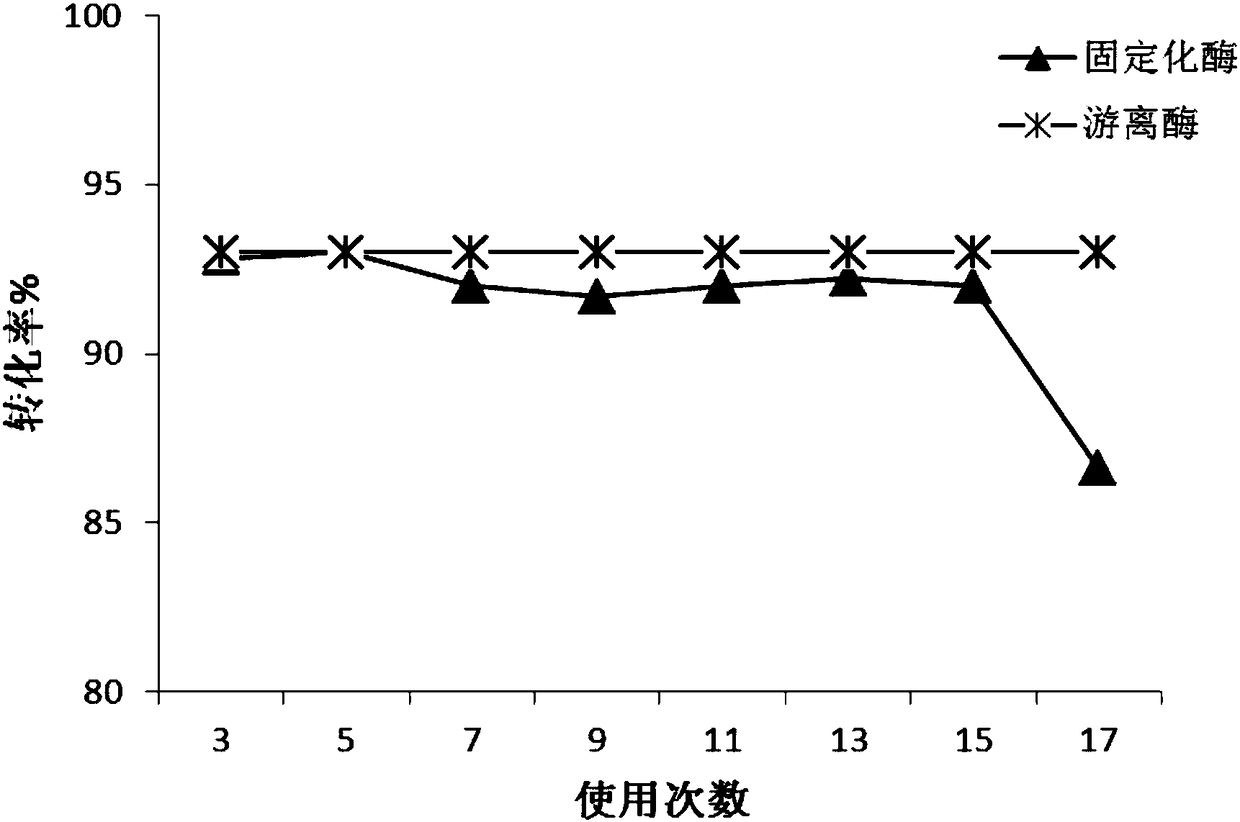
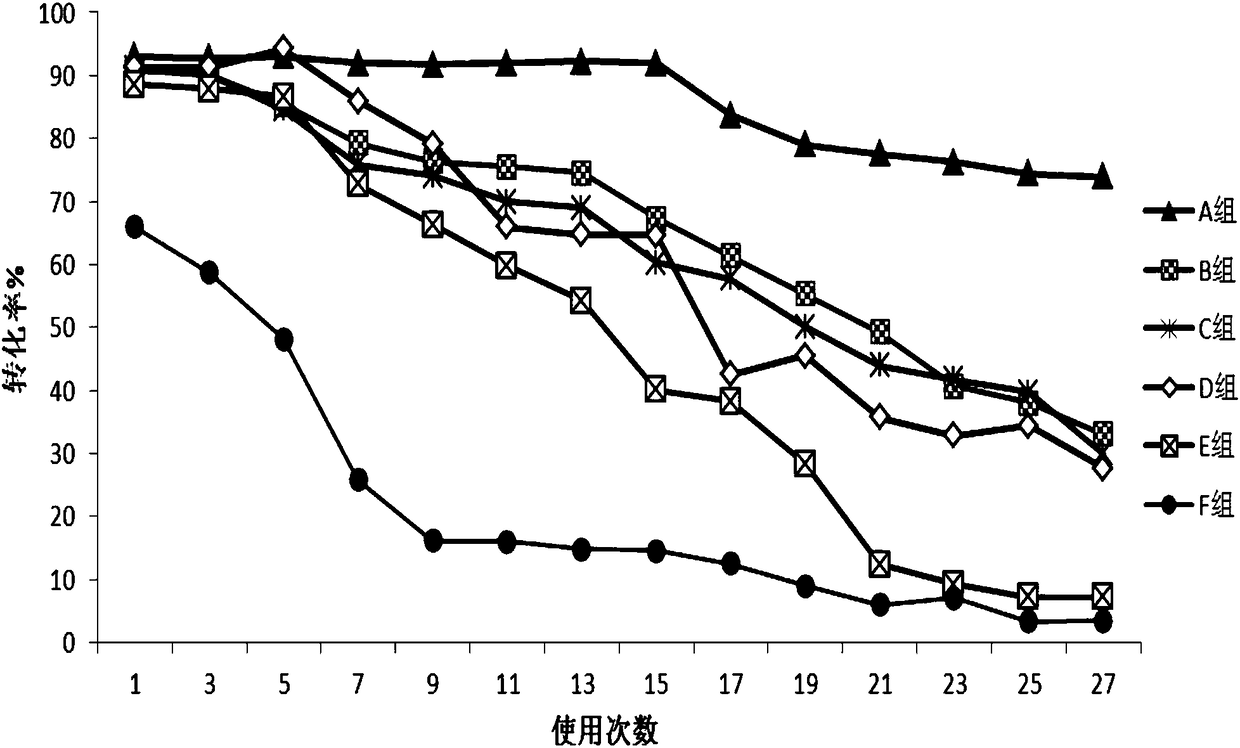
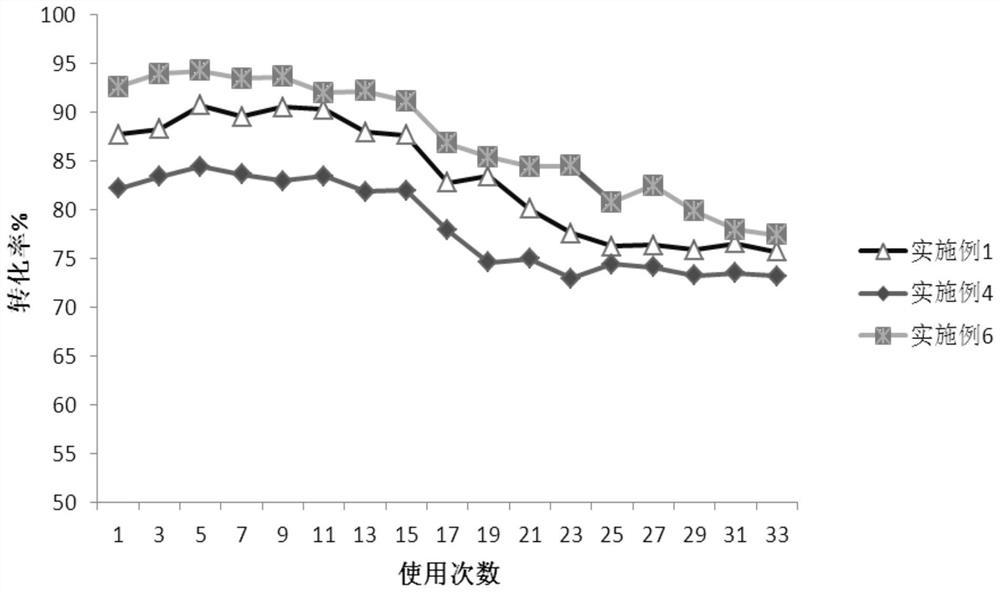
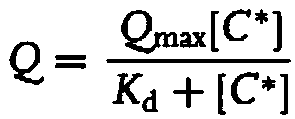Patents
Literature
34results about How to "High recovery rate of activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing freeze-dried human blood coagulation factor VIII
ActiveCN102228683AEasy to cleanGuaranteed stabilityPowder deliveryPeptide/protein ingredientsUltrafiltrationFreeze-drying
The invention discloses a method for preparing a freeze-dried human blood coagulation factor VIII. The method comprises the following process of: dissolving by taking water for injection, comprising 3-10 IU (International Unit) / ml of heparin, as a heparin sodium solution and cryoprecipitating; performing PEG (Polyethylene Glycol) precipitation and taking supernatant; performing centrifugal filtration; performing S / D (Solvent / Detergent) inactivation at the temperature of 24-26 DEG C; performing DEAE (Diethylaminoethyl) Sepharose Fast Flow chromatographic column balance, adsorption, washing andelution; performing molecular membrane ultrafiltration; preparing, removing bacteria, sub-packaging, freeze-drying and capping; and dry-heating at the temperature of 99.5-100.5 DEG C and inactivating. In the invention, the process method of combining the PEG precipitation and an ion exchange chromatography technology is adopted; the method is easy and convenient to operate; the F VIII active recovery rate is increased; miscellaneous proteins can be removed on a large scale; the product yield reaches over 60 percent; and the specific activity of the product reaches 5 IU / mg and is obviously greater than a value which is not less than 1 IU / mg stipulated in the pharmacopeia. Meanwhile, the PEG residue is 0.08g / L which is obviously less than the value which is less than or equal to 0.5 IU / mg stipulated in the pharmacopeia, so that Al<3+> residues in the final preparation of an Al(OH)3 gel method are avoided; the product has high purity and high safety; and the quality of the final product is obviously improved.
Owner:NANYUE BIOPHARMING
Silk nano granular of immobilized enzyme, and prepn. process thereof
InactiveCN1834240AGood stabilityGood biocompatibilityOn/in organic carrierMacromolecular non-active ingredientsChemistryWater soluble
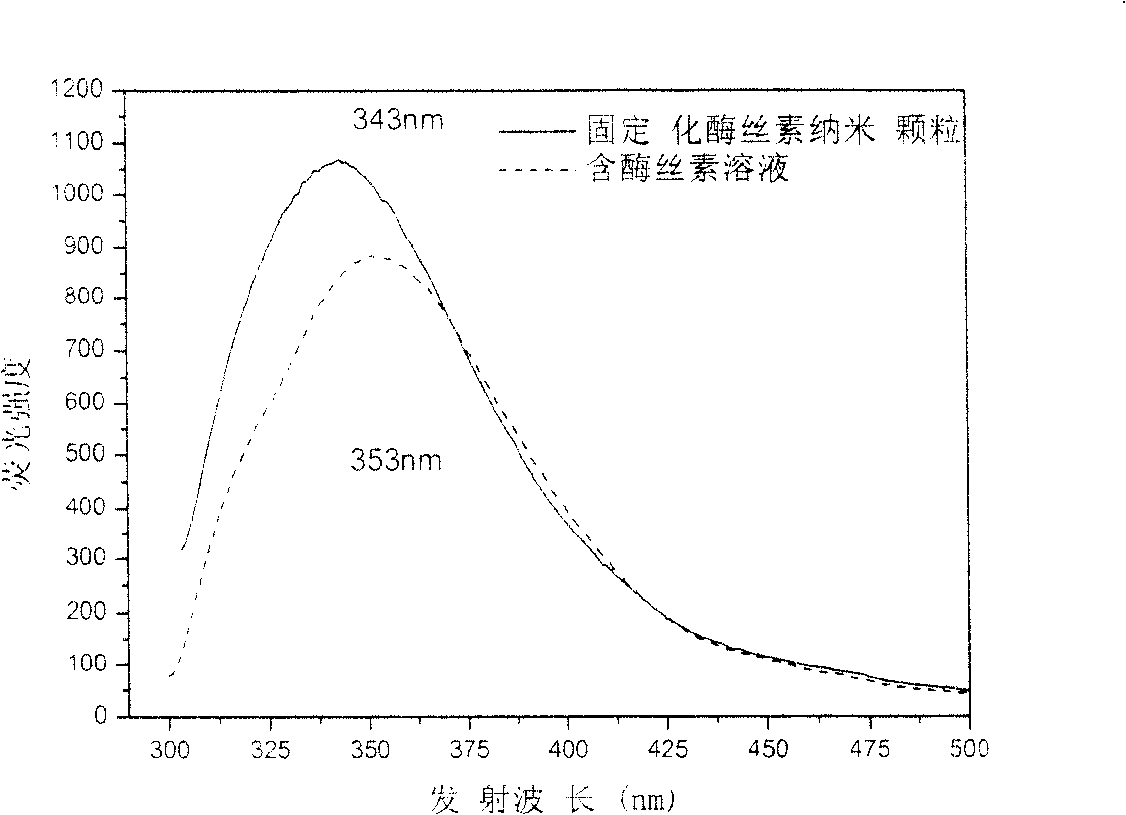
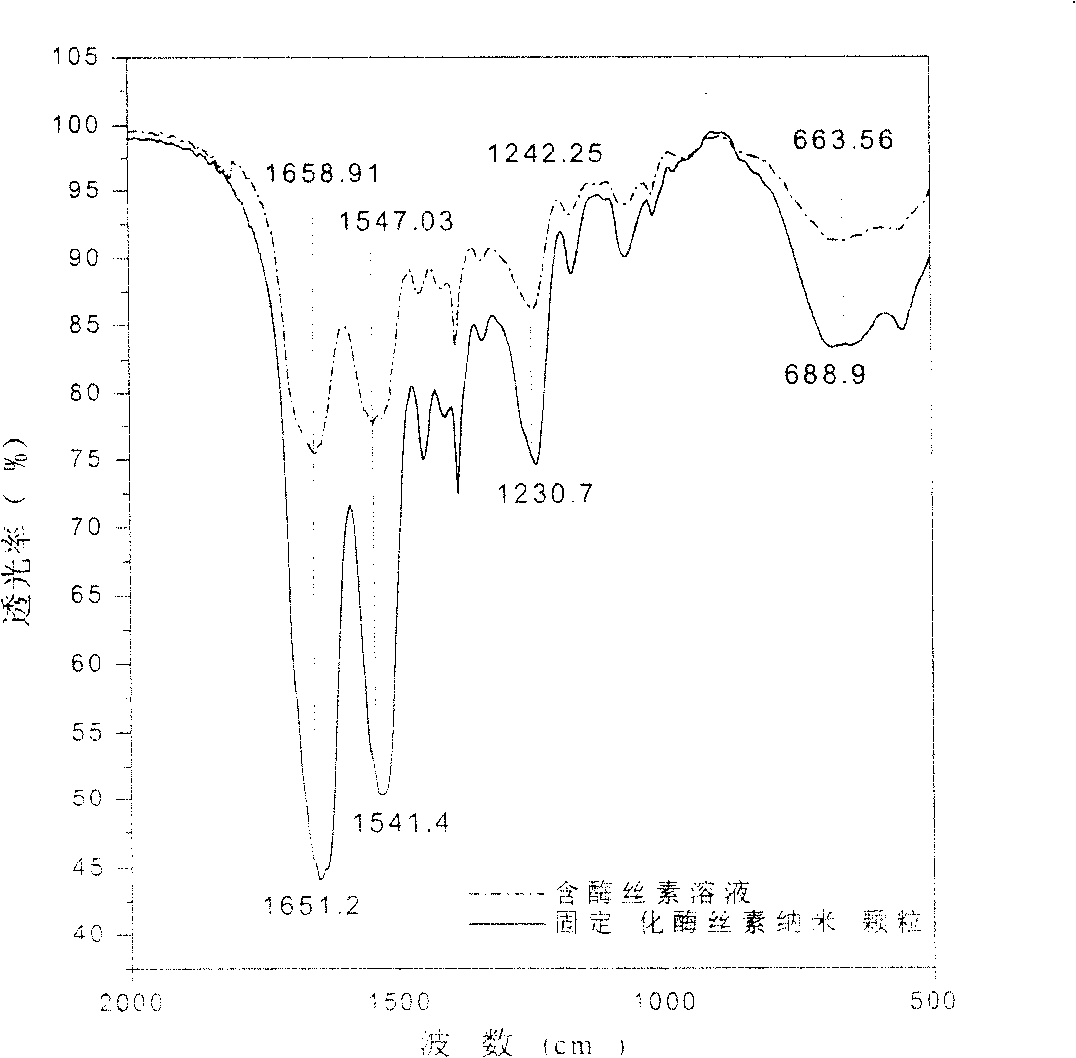
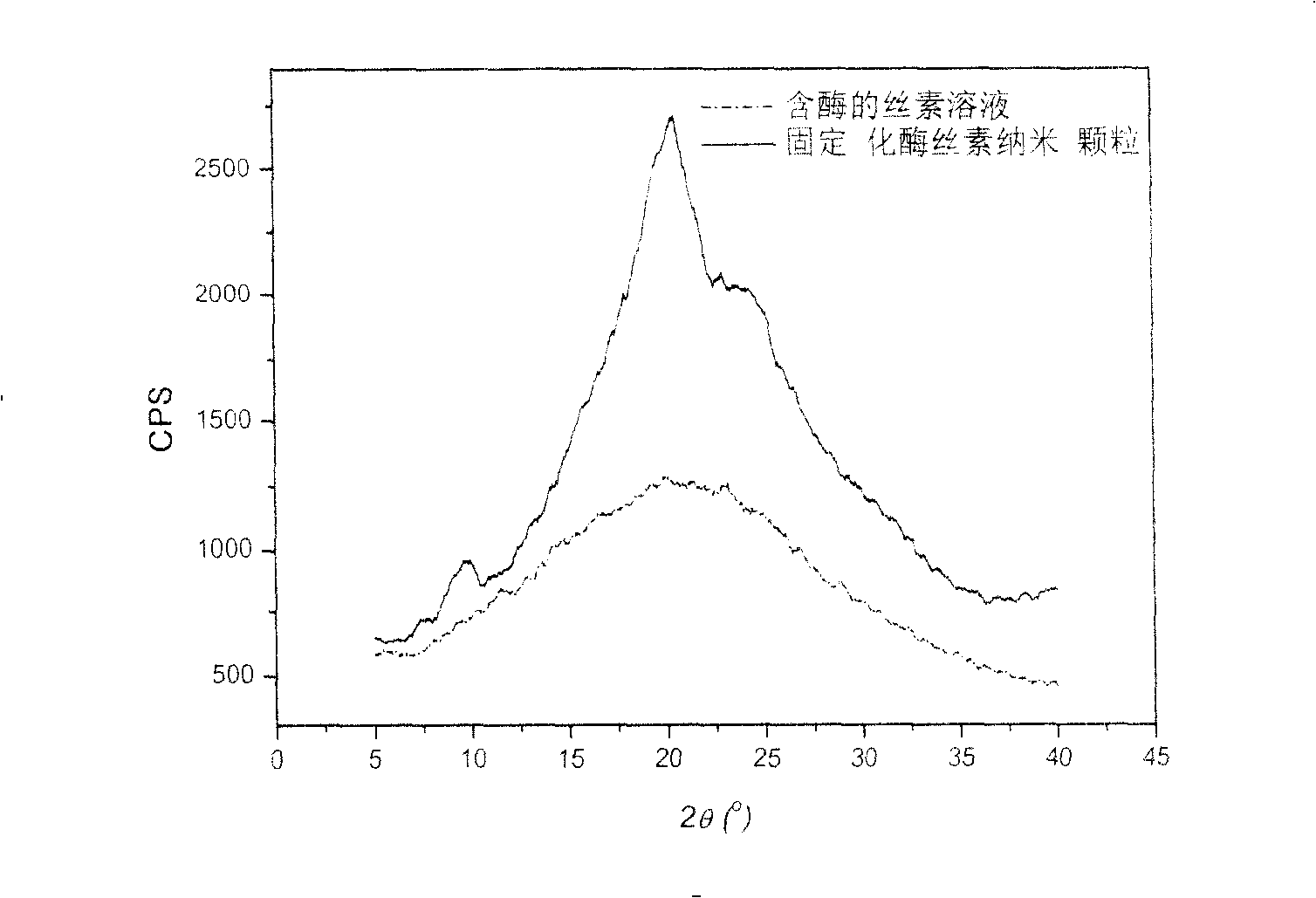
This invention discloses a method for manufacturing silk fibroin nanoparticles fixed with enzyme from silk fibroin, which comprises the steps of: (1) completely mixing the enzyme and regenerated silk fibroin solution; (2) injecting into a water-soluble organic solvent under high speed stirring to obtain white crystalline silk fibroin nanoparticles fixed with the enzyme; and (3) centrifuging or filtrating to remove the organic solvent to obtain crystalline silk fibroin nanoparticles fixed with the enzyme. The average particle size of the nanoparticles is 35-125 nm, and the activity recovery is as high as 70%. The fixed enzyme has a high thermal stability, and is not easy to be decomposed by proteinase, thus can largely reduce or even eliminate the immunogenicity of the enzyme. The nanoparticle fixed with the enzyme has important applications in sustained drug release, industrial enzyme reactor, food additives and cosmetics.
Owner:SUZHOU UNIV
Preparation of recyclable magnetic nanometer immobilized enzyme
InactiveCN101280298AIncrease profitThe method is simpleElectrical/wave energy microorganism treatmentOn/in inorganic carrierCross-linkCoprecipitation
The invention discloses a method for preparing reusable magnetic nanometer immobilized enzyme. The method comprises the following steps: nanometer Fe3O4 powder with surface on for amino group adsorption is prepared with a coprecipitation method; by using the surface groups, the nanometer Fe3O4 powder reacts with enzyme molecule amino group under the action of cross linking agent, and accomplishes cross linking process, then solid-liquid separation is performed under the action of external magnetic field, immobilized enzyme is recycled, and the magnetic nanometer immobilized enzyme which can be reused can be obtained. The magnetic nanometer immobilized enzyme of the invention has the advantages of high protein load capacity, high enzyme activity, convenient recycling and stable chemical property, and is suitable for the catalytic field, the degeneration of environment pollutant and the synthesizing process of energy material.
Owner:SHANDONG UNIV
Dry heat treatment method for human coagulation factor VIII preparation and dry heat treatment stabilizer
InactiveCN102430116AReduce lossesAchieve the inactivation effectPeptide/protein ingredientsMacromolecular non-active ingredientsFreeze-dryingFactor ii
The invention discloses a dry heat treatment method for a human coagulation factor VIII preparation. Before a freeze-drying step, the stabilizer is added into human coagulation factor VIII solution, wherein the stabilizer comprises human serum albumin, Ca2+ soluble salt, amino acid, sodium citrate and sodium chloride; and the concentration percentage of the human serum albumin is 0.5 to 5 percent and the concentration of Ca2+ is 1mmol / L. The invention also discloses the dry heat treatment stabilizer for the human coagulation factor VIII preparation. The dry heat treatment method is performed for 72 hours at 80 DEG C and is effective on both lipid envelop virus and non-lipid envelop virus. Usually, heat treatment is the last step. The method is low in cost and higher in maneuverability and controllability.
Owner:SHANGHAI XINXING MEDICINE
Method for preparing freeze-dried human blood coagulation factor VIII
ActiveCN102228683BImprove securityHigh recovery rate of activityPowder deliveryPeptide/protein ingredientsFreeze-dryingUltrafiltration
The invention discloses a method for preparing a freeze-dried human blood coagulation factor VIII. The method comprises the following process of: dissolving by taking water for injection, comprising 3-10 IU (International Unit) / ml of heparin, as a heparin sodium solution and cryoprecipitating; performing PEG (Polyethylene Glycol) precipitation and taking supernatant; performing centrifugal filtration; performing S / D (Solvent / Detergent) inactivation at the temperature of 24-26 DEG C; performing DEAE (Diethylaminoethyl) Sepharose Fast Flow chromatographic column balance, adsorption, washing andelution; performing molecular membrane ultrafiltration; preparing, removing bacteria, sub-packaging, freeze-drying and capping; and dry-heating at the temperature of 99.5-100.5 DEG C and inactivating. In the invention, the process method of combining the PEG precipitation and an ion exchange chromatography technology is adopted; the method is easy and convenient to operate; the F VIII active recovery rate is increased; miscellaneous proteins can be removed on a large scale; the product yield reaches over 60 percent; and the specific activity of the product reaches 5 IU / mg and is obviously greater than a value which is not less than 1 IU / mg stipulated in the pharmacopeia. Meanwhile, the PEG residue is 0.08g / L which is obviously less than the value which is less than or equal to 0.5 IU / mg stipulated in the pharmacopeia, so that Al<3+> residues in the final preparation of an Al(OH)3 gel method are avoided; the product has high purity and high safety; and the quality of the final product is obviously improved.
Owner:NANYUE BIOPHARMING
Biomimetic affinity purification method of para-aminophenamidine biomimetic affinity ligand
ActiveCN108144586ANon-destructiveSimple processIon-exchange process apparatusHydrolasesPurification methodsOrganic chemistry
The invention relates to a biomimetic affinity purification method of a para-aminophenamidine biomimetic affinity ligand. The biomimetic affinity purification method of the para-aminophenamidine biomimetic affinity ligand comprises preparation of a biomimetic affinity material as well as biomimetic affinity purification on metalloprotease (MP) by a para-aminophenamidine agarose gel column. The purification method is simple in flow, short in cycle and high in efficiency, and the purity is up to 98.7 percent.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Preparation method for magnetic sodium alginate-immobilized laccase
InactiveCN105505914ASimple and fast operationReduce manufacturing costOxidoreductasesOn/in organic carrierImmobilized enzymeLaccase
The invention discloses a preparation method for magnetic sodium alginate-immobilized laccase, and belongs to the technical field of immobilized enzyme preparation. According to the method, a laccase enzyme liquid is prepared by fermenting a laccase production strain, magnetic particles are mixed to immobilize the laccase enzyme liquid in combination of sodium alginate in an auxiliary manner, and drying is carried out after oscillation adsorption, so as to obtain the magnetic sodium alginate-immobilized laccase. The method is simple in process and low in cost, the activity of the prepared magnetic sodium alginate-immobilized laccase is 250 IU / mL or above, the total output is increased to 85-90%, and both the activity and the total output reach a higher level; moreover, through mixing of the nanoscale magnetic particles, the adsorption is more easy to realize, and separation is easy, so that the recovery rate of the immobilized laccase is improved, the stability is remarkably enhanced, and the laccase has excellent performance.
Owner:JIANGSU SHIKONG COATING
Preparation method of nano-magnetic chitin immobilized enzyme
InactiveCN1904043AStrong magneticHigh recovery rate of activityOn/in organic carrierLiquid ratioChitin formation
The present invention discloses a preparation method of nano magnetic chitosan immobilized enzyme. Said method includes the following steps: making nano magnetic chitosan particles whose surface contains acylchloro functional group be dispersed in enzyme-bearing solution, stirring them to make reaction for 2-8 hr at 20-25 deg.C, making solid-liquid separation in magnetic field, using phosphoric acid buffer solution to wash the magnetic precipitate for three times so as to obtain the required nano magnetic chitosan immobilized enzyme, the concentration of enzyme in enzyme-bearing solution is 0.01-0.1%. and the solid-liquid ratio of nano magnetic chitosan whose surface contains acylchloro functional group and enzyme-bearing solution is 1:50-1:200 g / ml.
Owner:SOUTH CHINA UNIV OF TECH
Separation and purification method of recombinant human interferon beta 1a
ActiveCN102140128AHigh purityHigh recovery ratePeptide preparation methodsDepsipeptidesUltrafiltrationChemistry
The invention discloses a separation and purification method of recombinant human interferon beta 1a. The separation and purification method comprises the following steps: 1) carrying out blue gel chromatography on a sample, collecting active peaks of the recombinant human interferon beta 1a; 2) carrying out ultrafiltration and desalination on a product obtained by the blue gel chromatography; 3)carrying out Q-Sepharose Fast Flow anion exchange chromatography on the ultrafiltration and desalination product, balancing a column by using 20mmol / / L Tris-HCl buffer liquid with the pH of 8.3-8.7, after sampling, eluting with the buffer liquid to a base line, and then eluting by using 20mmol / L NaCl-containing Tris-HCl buffer liquid with the pH of 8.3-8.7, and collecting the active peaks of the recombinant human interferon beta 1a; and 4) carrying out S-200 molecular sieve chromatography on the product obtained by the anion exchange chromatography so as to obtain the recombinant human interferon beta 1a. By using the purification method, a target protein has small possibility of inactivation, and the purity and biology specific activity of the target protein obtained by purification are high.
Owner:深圳未名新鹏生物医药有限公司
Sulfonated polystyrene microsphere immobilized alkaline protease and preparation method thereof
InactiveCN109182325ALarge specific surface areaSimple methodOn/in organic carrierPeptidasesAlkaline proteaseMicrosphere
The invention belongs to the technical field of immobilized enzymes and discloses sulfonated polystyrene microsphere immobilized alkaline protease and a preparation method thereof. The preparation method comprises the following steps: ultrasonically dispersing sulfonated polystyrene microspheres into distilled water; then adding into an alkaline protease solution and carrying out adsorption and immobilization reaction; and separating and washing a product to obtain the sulfonated polystyrene microsphere immobilized alkaline protease. The sulfonated polystyrene microsphere immobilized alkalineprotease prepared by the invention not only has high activity and recycling rate and a high loading amount, but also has a simple and rapid immobilization process; and the problem that the effect of atraditional immobilized enzyme is not ideal is solved.
Owner:SOUTH CHINA UNIV OF TECH +1
Method for removing endotoxin from influenza vaccine formulation
InactiveCN1621088AHigh removal rateStrong specificityAntiviralsPeptide preparation methodsFiltrationEndotoxin removal
The present invention is method of eliminating endotoxin from influenza vaccine. Affinity medium is prepared into affinity column or affinity membrane separator, which is used in processing influenza vaccine to eliminate endotoxin through static adsorption or filtration. The present invention features that the affinity medium has polyethylene imine as affinity ligand and nitrogen content of 0.75-7.0 %. The present invention has high endotoxin eliminating rate, high specificity and high active influenza vaccine recovering rate, and is suitable for production.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Process for preparing human prothrombin complex concentrate by adopting flow adsorption method
ActiveCN108441490ALow purityHigh purityBioreactor/fermenter combinationsBiological substance pretreatmentsProthrombin complex concentrateDynamic balance
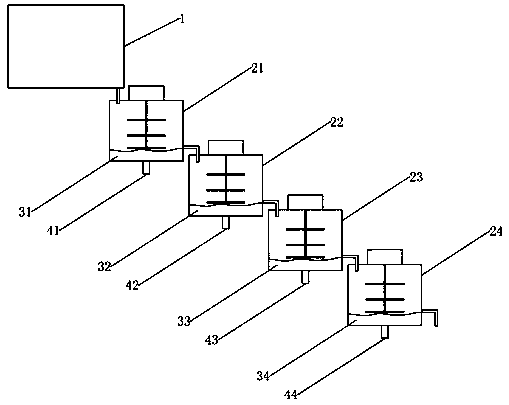
The invention discloses a process for preparing a human prothrombin complex concentrate by adopting a flow adsorption method. The process is characterized in that a plurality of cylindrical containerswith stirrers are connected in series to form a natural flowing way; gels which are balanced in advance are placed in the various containers; a certain batch of to-be-processed blood plasma is storedabove the first container; the gels are injected quantitatively firstly in the first container, then the gels are stirred and adsorbed for a unit time, and the adsorbed blood plasma is discharged into the second container at a certain flow speed; simultaneously, other to-be-processed blood plasma flows into a first adsorption tank at the same flow speed, so that dynamic balance of the blood plasma quantity in the first container is kept, and at this time, adsorption and separation of the blood plasma in the first container are carried out simultaneously; by analogy, after the all blood plasmapasses through, outlets of the various containers are closed, and washing and elution on the gels are carried out. The blood plasma quantity is not limited by the volume of the containers, the process is easily controlled, an FIX activety recycling ratio is higher, and the risks of leakage of the gels and crossed pollution are avoided.
Owner:华润博雅生物制药集团股份有限公司
Immobilized cutinase and preparation method and application thereof in removal of phthalic acid esters in water
InactiveCN103756991AGood biocompatibilityLarge adsorption capacityWater contaminantsOn/in inorganic carrierCutinaseBiocompatibility Testing
The invention relates to the field of enzyme immobilization, and in particular relates to an immobilized cutinase and a preparation method and an application thereof in removal of phthalic acid esters in water. The immobilized cutinase is fixed on a porous gold nanomaterial through a physical absorption function by taking the porous gold nanomaterial as a carrier, wherein the adsorption quantity of the cutinase on the porous gold nanomaterial is more than 30mg / g; the bore diameter of the porous gold nanomaterial is between 40nm and 50nm. The preparation method comprises the following steps: adding the porous gold nanomaterial into a cutinase solution; adsorbing in an oscillation manner at 45 DEG C to 55 DEG C; and performing washing, freezing and vacuum drying, thereby obtaining the immobilized cutinase. The immobilized cutinase can be used for degrading the phthalic acid esters in the water, and is good in biocompatibility, high in adsorption quantity and good in adsorption stability.
Owner:HUNAN UNIV
Natural active protein and peptide separating and purifying process
The present invention is natural active protein and peptide separating and purifying process. The natural material with rich protein is processed through defatting, cleaning, homogenizing, mixing with deionized water inside a bioreactor regulating pH to 5-8, heating to 70-90 deg.c, maintaining and stirring for 10-30 min, quick cooling to 30-50 deg.c and mixing with composite proteinase for enzymolysis to obtain mixture of protein and peptide. Through processing with polyethersulfone superlow protein adsorbing membrane via OMEGA technological process treatment and tangent flow superfiltering to grade according to molecular weight, purifying with ion exchange chromatographic mode, etc. natural active protein and peptide products with different molecular and different molecular structure and obtained.
Owner:广州瑞谷生物技术有限公司
Silk nano granular of immobilized enzyme, and prepn. process thereof
InactiveCN100427593CReduce or eliminate immunogenicityExtended half-lifeOn/in organic carrierMacromolecular non-active ingredientsFood additiveBiotechnology
This invention discloses a method for manufacturing silk fibroin nanoparticles fixed with enzyme from silk fibroin, which comprises the steps of: (1) completely mixing the enzyme and regenerated silk fibroin solution; (2) injecting into a water-soluble organic solvent under high speed stirring to obtain white crystalline silk fibroin nanoparticles fixed with the enzyme; and (3) centrifuging or filtrating to remove the organic solvent to obtain crystalline silk fibroin nanoparticles fixed with the enzyme. The average particle size of the nanoparticles is 35-125 nm, and the activity recovery is as high as 70%. The fixed enzyme has a high thermal stability, and is not easy to be decomposed by proteinase, thus can largely reduce or even eliminate the immunogenicity of the enzyme. The nanoparticle fixed with the enzyme has important applications in sustained drug release, industrial enzyme reactor, food additives and cosmetics.
Owner:SUZHOU UNIV
Phospholipid nano-disk chromatography medium and preparation method and application thereof
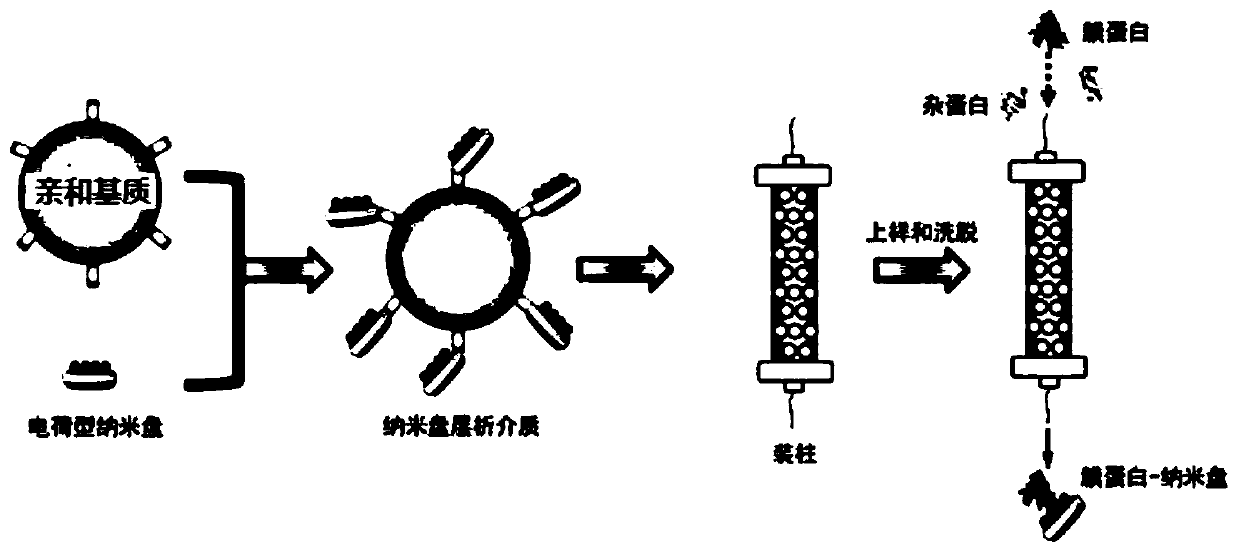
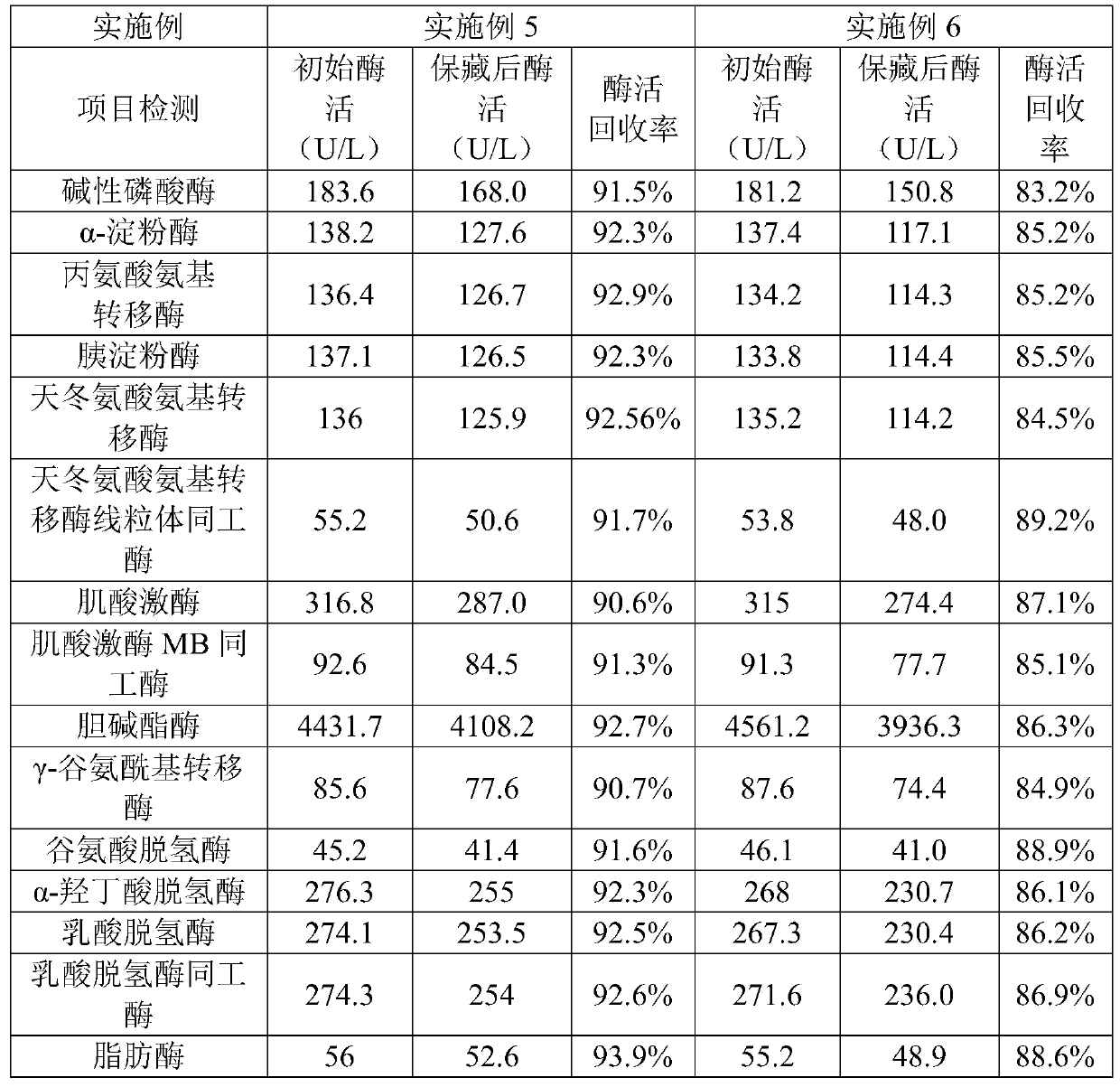
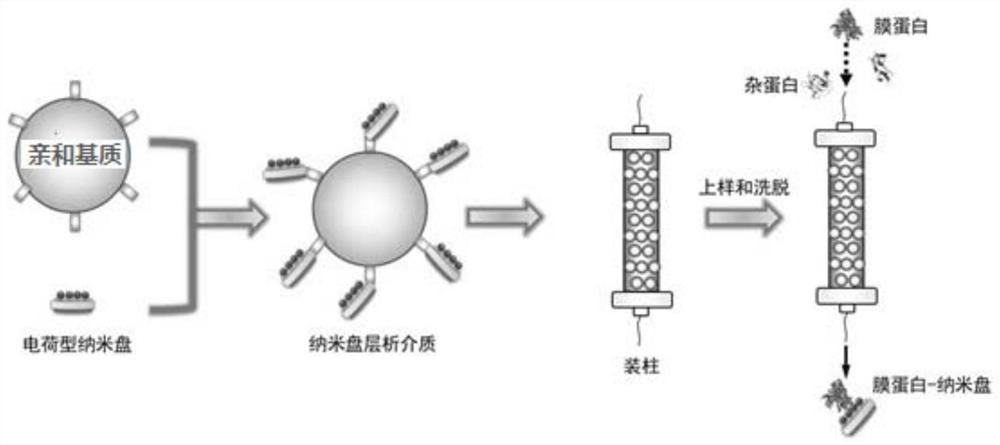
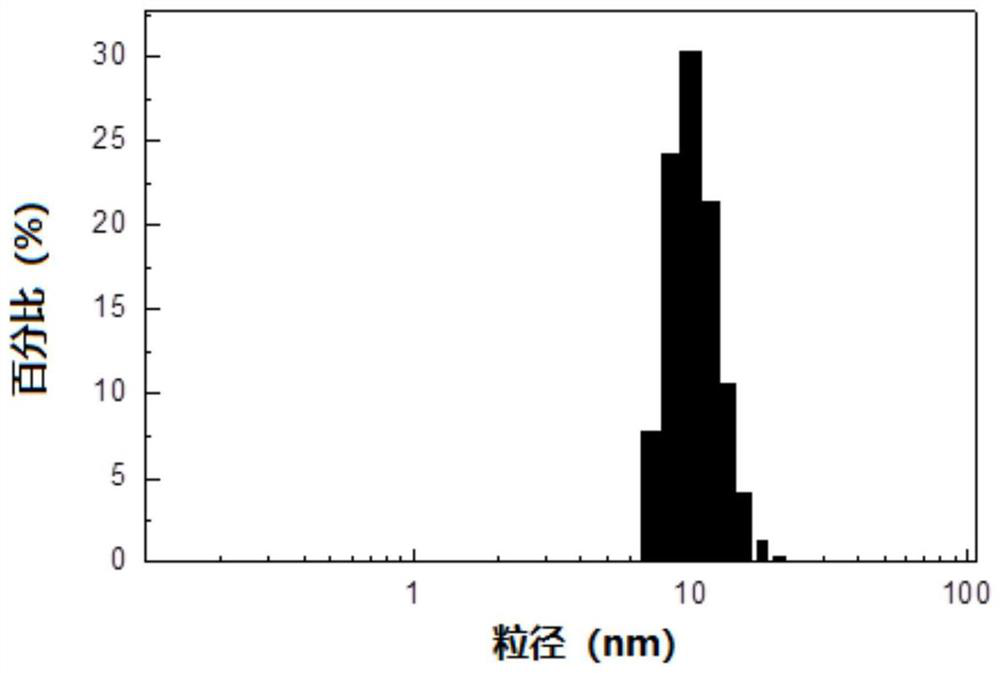
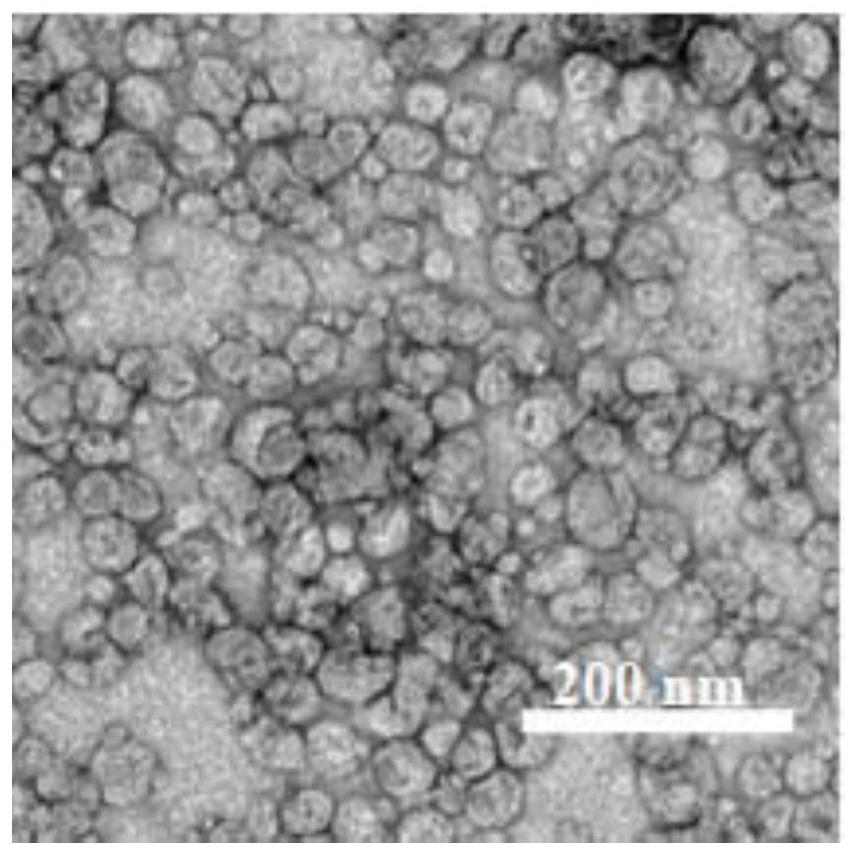
ActiveCN110292916AGuaranteed protection functionFunction to purify proteinOther chemical processesDepsipeptidesPhospholipidProtein activity
The invention relates to a phospholipid nano-disk chromatography medium and a preparation method and application thereof. The phospholipid nano-disk chromatography medium comprises a charged phospholipid nano disk and an affinity matrix; the charged phospholipid nano disk comprises phospholipid and a film scaffold protein with an affinity label; the charged phospholipid nano disk is connected withan affinity matrix through the affinity label. The phospholipid nano-disk chromatography medium has both binding capacity and protection capacity to film proteins, so that the actual system containing the film proteins can be purified and reconstructed on a chromatography column in one step; and the phospholipid nano-disk chromatography medium has reversibility and regeneration performance; the phospholipid nano-disk chromatography medium solves the problems of serious protein activity loss, long and complicated steps and time-consuming and labor-consuming operation in the traditional film protein research technology, is simple and efficient to operate, improves the film protein activity recovery rate by more than 20 times, and shortens the treatment time by more than 95%.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +1
Substrate for biochemical calibrator and biochemical calibrator
PendingCN110954380AIncrease viscosityMaintain spatial structurePreparing sample for investigationBiological testingBiotechnologyMagnesium salt
The invention is applicable to the technical field of biochemistry, in particular to a calibrator for a biochemical calibrator and the biochemical calibrator. Each liter of the matrix for the biochemical calibrator comprises the following components of 10 to 100 mmol of a pH regulator, 2-50 g of a saccharide protective agent, 2-100 g of a polymer protective agent, 5-50 g of a protein protective agent, 0.5 to 5 grams of surfactant, 5 to 20 millimoles of amino acid, 0.02 to 50 millimoles of antioxidant, 0.05 to 2 millimoles of protease inhibitor, 5 to 30 grams of alcohol organic solvent, 0.2 to0.5 gram of preservative, 8.5 to 9.5 grams of sodium chloride and 0.1 to 5 millimoles of magnesium salt and / or zinc salt. According to the calibrator for the biochemical calibrator, when the analysiscomponents are added into the matrix for the biochemical calibrator, the components in the matrix for the biochemical calibrator can play a better role in protecting the analysis components, particularly, the structural variation caused by water loss of the analysis components in the freeze-drying process can be avoided, and the effect of the analysis components is effectively reserved.
Owner:NINGBO RUI BIO TECH
A kind of phospholipid nano disc chromatography medium and its preparation method and application
ActiveCN110292916BGuaranteed protection functionFunction to purify proteinOther chemical processesDepsipeptidesNanodiscPhospholipid
The invention relates to a phospholipid nano-disk chromatography medium and a preparation method and application thereof. The phospholipid nano-disk chromatography medium comprises a charged phospholipid nano disk and an affinity matrix; the charged phospholipid nano disk comprises phospholipid and a film scaffold protein with an affinity label; the charged phospholipid nano disk is connected withan affinity matrix through the affinity label. The phospholipid nano-disk chromatography medium has both binding capacity and protection capacity to film proteins, so that the actual system containing the film proteins can be purified and reconstructed on a chromatography column in one step; and the phospholipid nano-disk chromatography medium has reversibility and regeneration performance; the phospholipid nano-disk chromatography medium solves the problems of serious protein activity loss, long and complicated steps and time-consuming and labor-consuming operation in the traditional film protein research technology, is simple and efficient to operate, improves the film protein activity recovery rate by more than 20 times, and shortens the treatment time by more than 95%.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +1
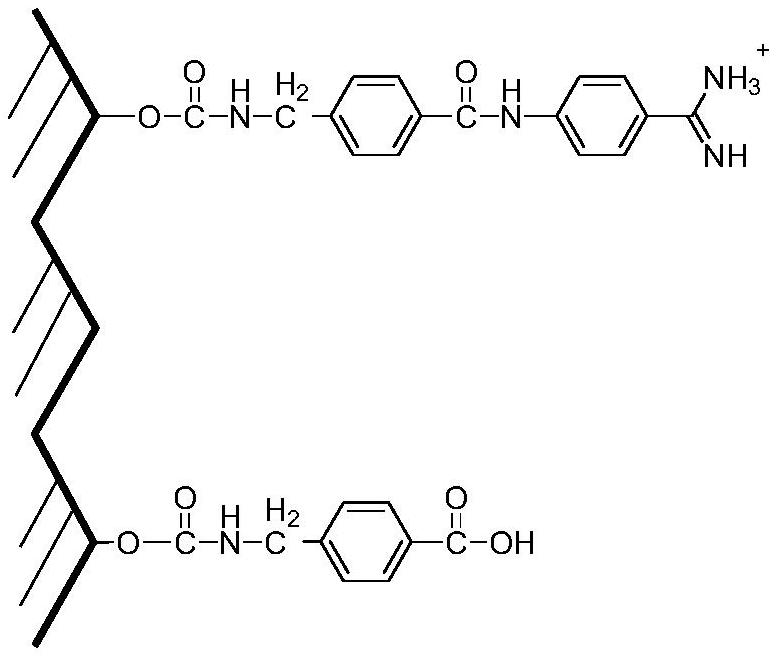
A kind of affinity chromatography material for purifying urokinase and its preparation method and application
ActiveCN111957073BEasy to separate and purifyGood activity recoverySolid sorbent liquid separationPeptidasesKinaseHigh activity
The invention provides an affinity chromatography filler for purifying urokinase and its preparation method and application. The invention belongs to the field of biochemical purification. The invention uses aminotoluic acid and 4-aminobenzamidine dihydrochloride in the implementation process Sepharose CL-6B was modified to obtain a Sepharose CL-6B-aminomethylbenzoic acid-p-AB filler, which can better purify and separate high-molecular-weight urokinase and low-molecular-weight urokinase, and the activity recovery rate is high, both in More than 95%, the recovery effect is good.
Owner:武汉人福药业有限责任公司
A high-efficiency hydrophobic interaction chromatography medium with benzylamine as a ligand, its preparation method and its application in protein refolding and purification
ActiveCN109078628BImprove renaturation efficiencyHigh recovery rate of activityOther chemical processesSolid sorbent liquid separationEpoxyStructural formula
The invention discloses a high-efficiency hydrophobic interaction chromatographic medium with amine as a ligand, and its structural formula is:; the invention also discloses a preparation method of the high-efficiency hydrophobic interaction chromatographic medium, which includes the following steps: (1) Silica gel activation; (2) Preparation of epoxy silica gel; (3) Preparation of high-efficiency hydrophobic interaction chromatography medium by epoxy ring opening; at the same time, the present invention also discloses the refolding and reconstitution of the high-efficiency hydrophobic interaction chromatography medium with recombinant streptavidin The application of purification has high renaturation efficiency, high activity recovery rate, and high purity of recombinant streptavidin after renaturation.
Owner:NORTHWEST UNIV
A temperature-controlled phase change material microcapsule carrier immobilized enzyme and its preparation method
ActiveCN105950606BWide temperature rangeImprove thermal stabilityOn/in organic carrierOn/in inorganic carrierChemical couplingMaterials science
The invention discloses a temperature-regulated phase material microcapsule carrier immobilized enzyme and a preparation method for the same, and relates to the technical field of biology. The preparation method comprises the following steps: preparing a microcapsule carrier obtained by cladding an organic phase change material with a crystalline titanium dioxide / magnetic particle composite material, performing carboxylation modification on the microcapsule carrier, and implementing enzyme immobilization by adopting a chemical coupling method, so as to finally obtain the temperature-regulated phase material microcapsule carrier immobilized enzyme. According to the temperature-regulated phase material microcapsule carrier immobilized enzyme and the preparation method for the same, heat can be stored and released by the microcapsule carrier of the immobilized enzyme through a phase change material capsule core, so that micro-environmental temperature around the carrier can be regulated, the temperature application range of enzymatic reaction can be widened, and the heat stability, the storage stability and the cycling stability of the immobilized enzyme can be enhanced. In addition, the temperature-regulated phase change material microcapsule carrier can be used for immobilizing different types of enzymes, and is wide in application range.
Owner:BEIJING UNIV OF CHEM TECH
Affinity chromatography filler for purifying urokinase and preparation method and application thereof
ActiveCN111957073AEasy to separate and purifyGood activity recoverySolid sorbent liquid separationPeptidasesSepharoseAminomethylbenzoic acid
The invention provides an affinity chromatography filler for purifying urokinase and a preparation method and application of the affinity chromatography filler. The invention belongs to the field of biochemical purification. In the implementation process of the preparation method, aminomethylbenzoic acid and 4-aminobenzamidine dihydrochloride are used for modifying Sepharose CL-6B, the Sepharose CL-6B-aminomethylbenzoic acid-p-AB filler can better purify and separate macromolecular urokinase and low molecular urokinase, the activity recovery rate is high and is 95% or above, and the recovery effect is good.
Owner:武汉人福药业有限责任公司
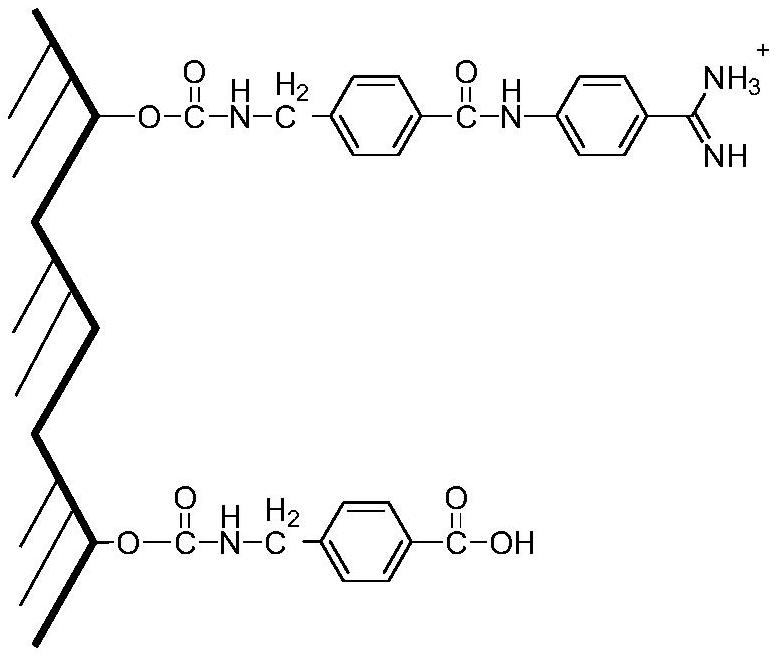
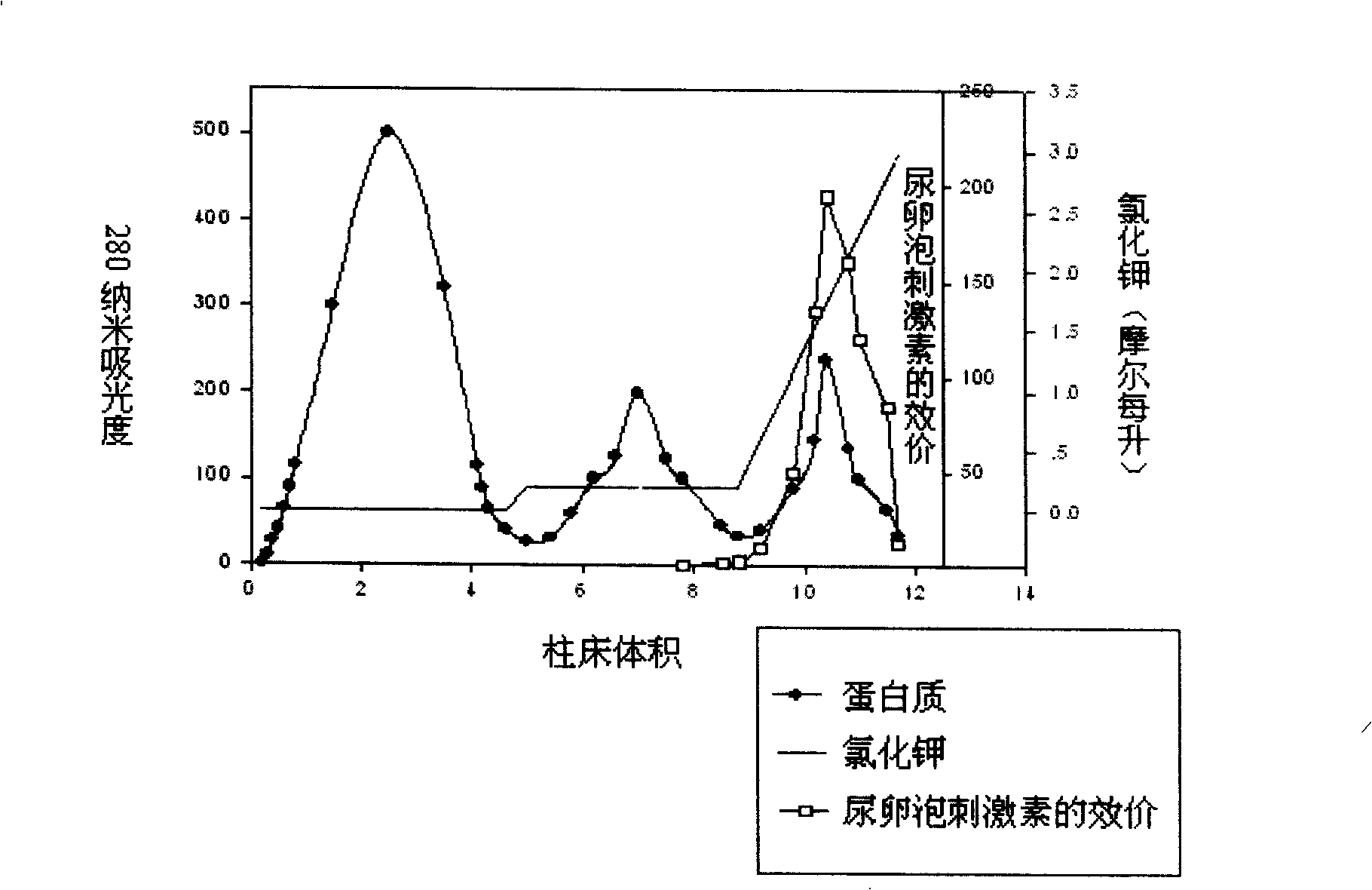
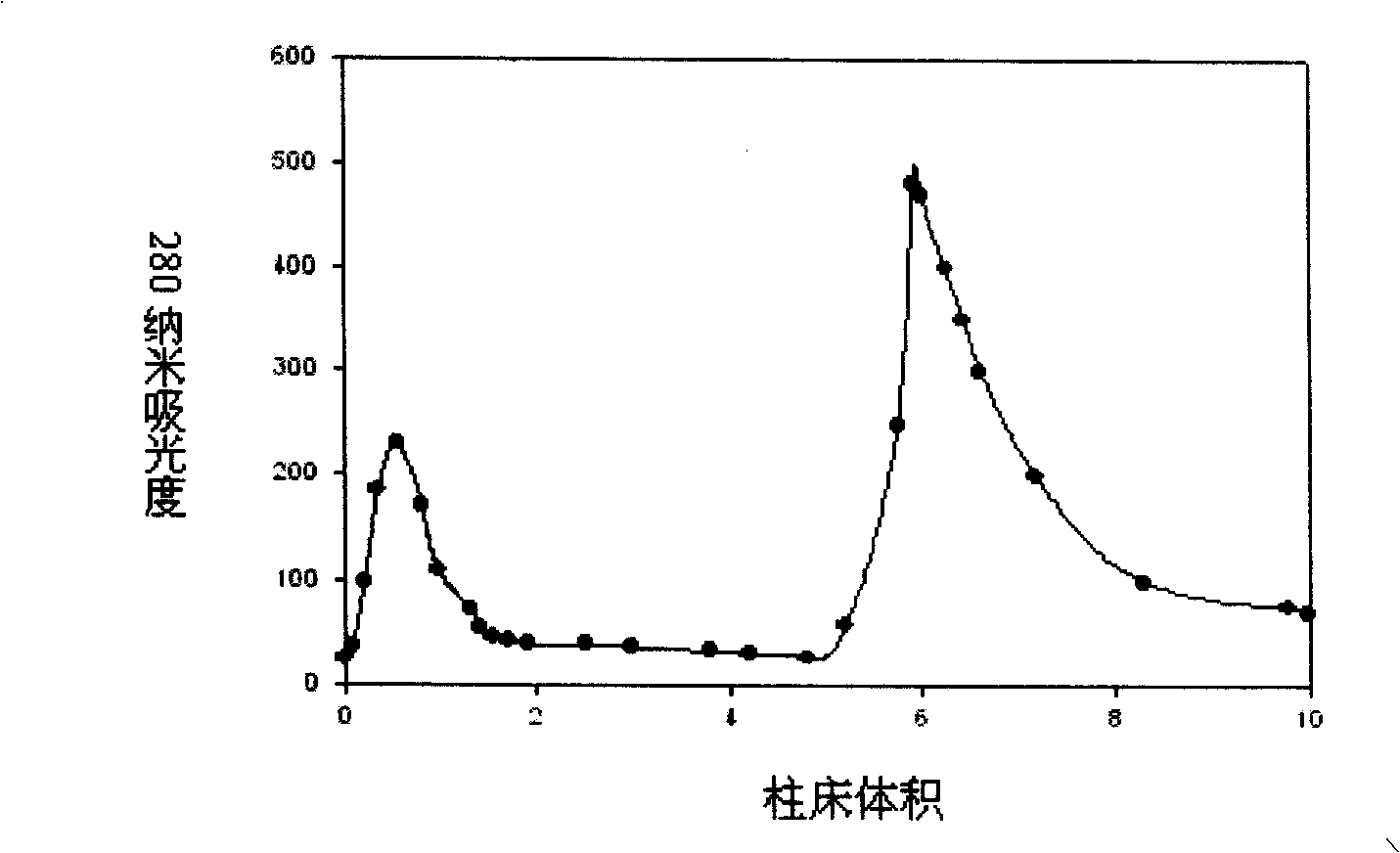
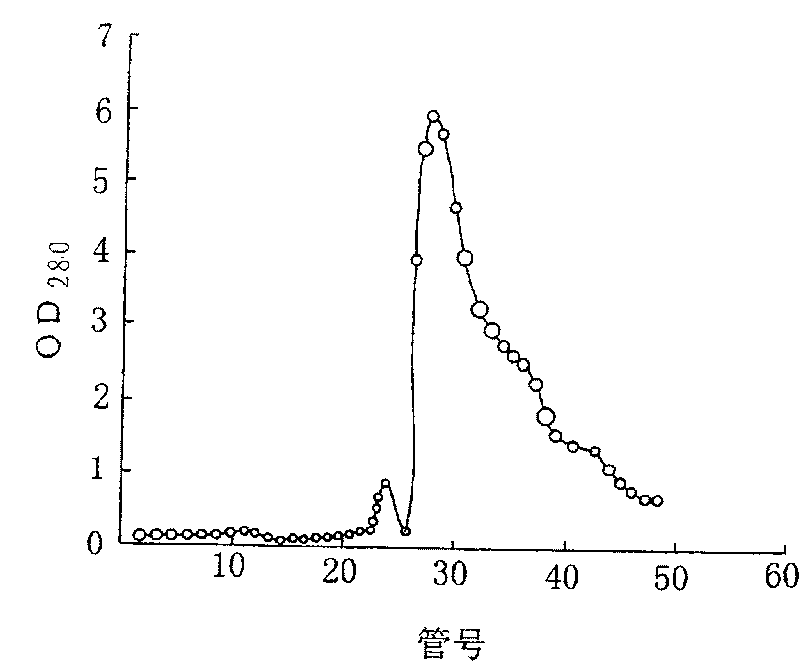
Purifying and producing process for high purity follicle stimulating hormone in urine
InactiveCN100417663CHigh purityHigh recovery rate of activityDepsipeptidesPeptide preparation methodsFollicle-stimulating hormoneIon exchange
During the purification and production of high purity uric follicle stimulating hormone, red azo dye is coupled to Sepharose 4B as the medium of affinity separating and purifying uric follicle stimulating hormone, and through dye affinity chromatography, QAE ion exchange chromatography and other technological steps, high purity uric follicle stimulating hormone product is obtained from uric gonadotropin from post-menopause. The product produced based on the said technological process has biological potency not lower than 200 IU / mg and FSH / LH higher than 100, is white and soluble, and may be used directly in producing injection.
Owner:NANCHANG WANHUA BIOCHEM PHARMA
Extraction process of camel colostrum immune globulin IgA, IgG.
Owner:甘肃省华龙农业开发有限公司
Method of eliminating endotoxin from interferon preparation
InactiveCN1324048CHigh removal rateHigh recovery rate of activityPeptide preparation methodsInterferonsFiltrationPorous medium
The present invention relates to a production of recombinant protein medicine, in the concrete, it relates to a method for removing endotoxin from interferon preparation. It is characterized by that the chitosan or desoxycholate sodium or hexanediamine is used as affinity ligand, and can be covalently combined on the porous medium, then a dynamic filtration or static adsorption mode can be used to make operation so as to remove endotoxin from interferon preparation, in which the quantity of affinity ligand on the porous medium is 0.02%-3% nitrogen content. Said invention is high in endotoxin removing rate, high in interferon activity recovery and good in specificity.
Owner:BEIJING KAWIN TECH SHARE HLDG
Biomimetic affinity purification method of p-aminobenzamidine biomimetic affinity ligand
ActiveCN108144586BNon-destructiveSimple processIon-exchange process apparatusHydrolasesP-aminobenzamidineSepharose
The invention relates to a bionic affinity purification method of p-aminobenzamidine bionic affinity ligand, including the preparation of bionic affinity materials and the bionic affinity of p-aminobenzamidine agarose gel column to metalloprotease MP purification. The purification method has the advantages of simple flow, short period, high efficiency and high purity of 98.7%.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Immobilized trypsin as well as preparation method and application thereof
ActiveCN108239634AImprove stabilityImprove bindingOn/in organic carrierPeptidasesOrganic solventMicrosphere
The invention discloses immobilized trypsin as well as a preparation method and application thereof. The preparation method comprises the steps of: with a large-aperture polymeric microsphere as an immobilized enzyme carrier, completely cleaning, activating functional groups of the microsphere, mixing the microsphere with a trypsin solution, carrying out vibration reaction so as to generate covalent binding by virtue of enzyme molecules and the functional groups on the surface of the carrier, standing, adjusting the pH value to be 9 or over, and carrying out maintenance for a certain period oftime, so as to obtain the immobilized trypsin. The preparation method is stable in process and mild in reaction conditions; the protein immobilization rate of the prepared immobilized trypsin can reach 92.7%; the activity recovery rate is high, and the fluctuation is small; the stability of the immobilized trypsin in an organic solvent medium is substantially improved; and meanwhile, the pH valueapplication range is relatively wide, the acid and alkali resistances are relatively good, and the immobilized trypsin can still preserve very good activity in an environment with a pH value of 2-12and has relatively wide application range.
Owner:ZHUHAI UNITED LAB
A kind of immobilized trypsin and its preparation method and application
ActiveCN108239634BImprove stabilityImprove bindingOn/in organic carrierPeptidasesMicrosphereOrganosolv
The invention discloses an immobilized trypsin and its preparation method and application. The present invention uses macroporous polymer microspheres as immobilized enzyme carriers, cleans them, activates their functional groups, mixes them with trypsin solution, and vibrates to react, so that the enzyme molecules and the functional groups on the surface of the carrier are covalently bonded. , let it stand still, and then adjust the pH value to above 9, and maintain it for a certain period of time to obtain immobilized trypsin. The preparation method has stable process and mild reaction conditions; the immobilized trypsin protein immobilization rate can reach 92.7%; the activity recovery rate is high and the fluctuation is small; the stability in the organic solvent medium is greatly improved; at the same time, the pH value is suitable The range is wider, the acid and alkali resistance is better, the immobilized enzyme can still maintain good activity in the environment of pH 2-12, and the application range is wider.
Owner:ZHUHAI UNITED LAB
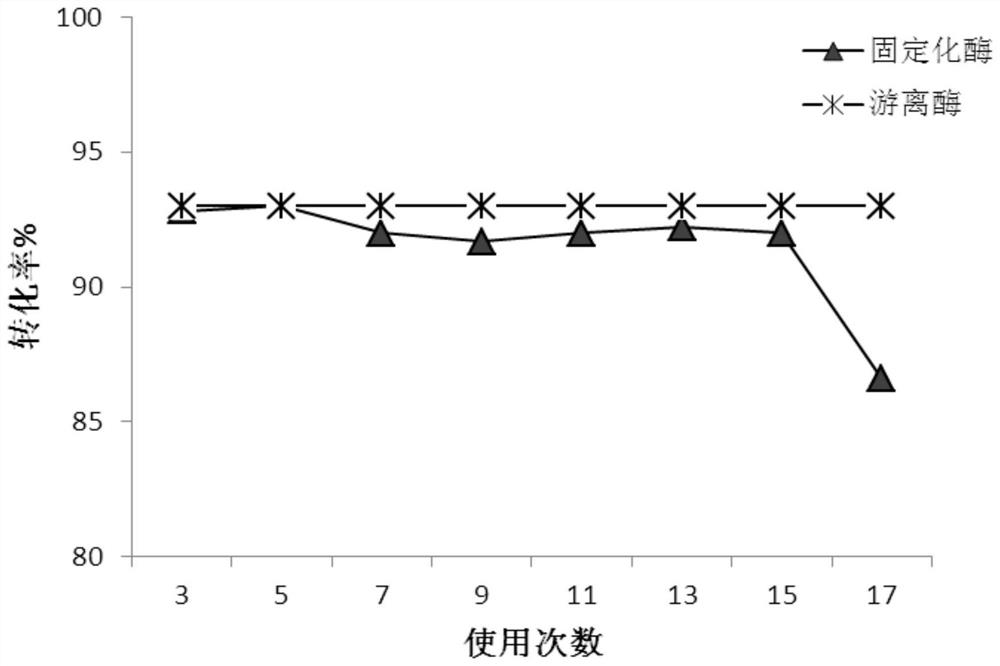
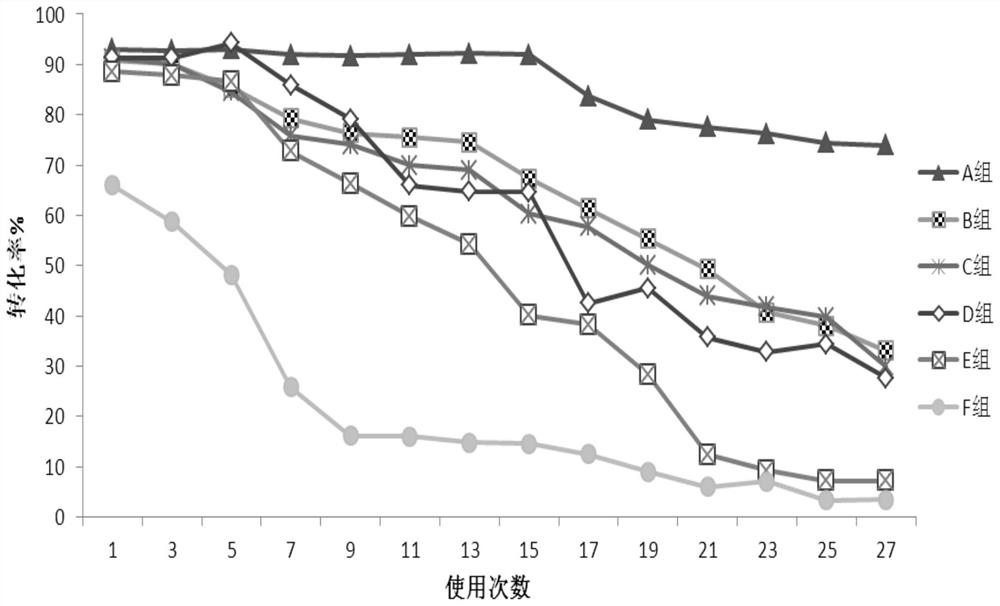
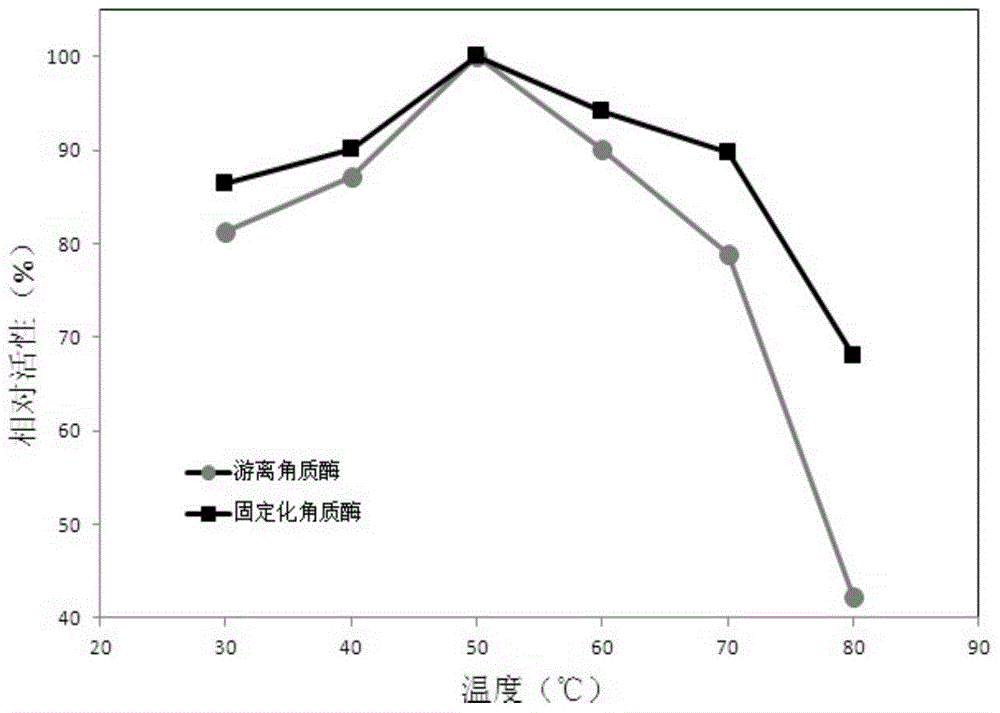
Efficient preparation method of papain preparation
The invention relates to an efficient preparation method of a papain preparation. The method comprises the following specific operation steps: S1, preparing a papain preparation crude product; S2, preparing a papain crude enzyme product solution and a papaya lipase crude enzyme product; S3, preparing a papaya lipase preparation; and S4, isolating and purifying papain. According to the efficient preparation method of the papain preparation disclosed by the embodiment of the invention, the papain and the papaya lipase can be obtained simultaneously by isolation and purification of papaya primary pulp, complete utilization of resources is realized, the preparation steps are simple, the design is reasonable, the operation is simple, the influence on the activity of the enzymes in the preparation process is relatively small, the prepared papain and papaya lipase are high in purity, high in activity and high in enzyme activity recovery rate; and reagents can be recycled after isolation is performed with a strong cation exchange method, so that the reagents are repeated used, the cost is effectively reduced, the preparation can be expanded, and the industrial requirements are met.
Owner:HAINAN UNIVERSITY
Immobilized cutinase, preparation method and application of removing phthalates in water
InactiveCN103756991BGood biocompatibilityLarge adsorption capacityWater contaminantsOn/in inorganic carrierCutinaseBiocompatibility Testing
The invention relates to the field of enzyme immobilization, and in particular relates to an immobilized cutinase and a preparation method and an application thereof in removal of phthalic acid esters in water. The immobilized cutinase is fixed on a porous gold nanomaterial through a physical absorption function by taking the porous gold nanomaterial as a carrier, wherein the adsorption quantity of the cutinase on the porous gold nanomaterial is more than 30mg / g; the bore diameter of the porous gold nanomaterial is between 40nm and 50nm. The preparation method comprises the following steps: adding the porous gold nanomaterial into a cutinase solution; adsorbing in an oscillation manner at 45 DEG C to 55 DEG C; and performing washing, freezing and vacuum drying, thereby obtaining the immobilized cutinase. The immobilized cutinase can be used for degrading the phthalic acid esters in the water, and is good in biocompatibility, high in adsorption quantity and good in adsorption stability.
Owner:HUNAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com