Immobilized trypsin as well as preparation method and application thereof
A technology of trypsin and immobilized enzyme carrier, which is applied in the field of immobilized trypsin and its preparation, which can solve the problems of insufficient stability, large fluctuation of activity recovery rate, and large loss of enzyme activity, so as to achieve the effect of improving stability and improving stability performance, stability enhancement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
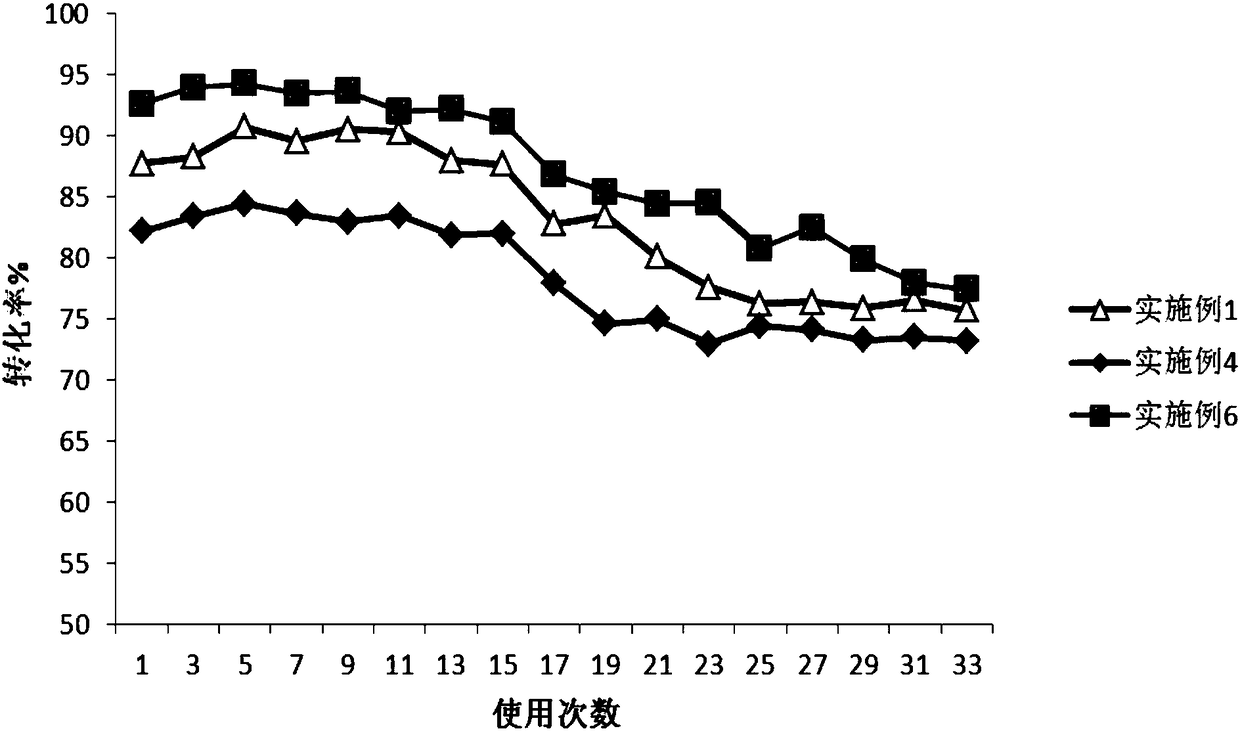
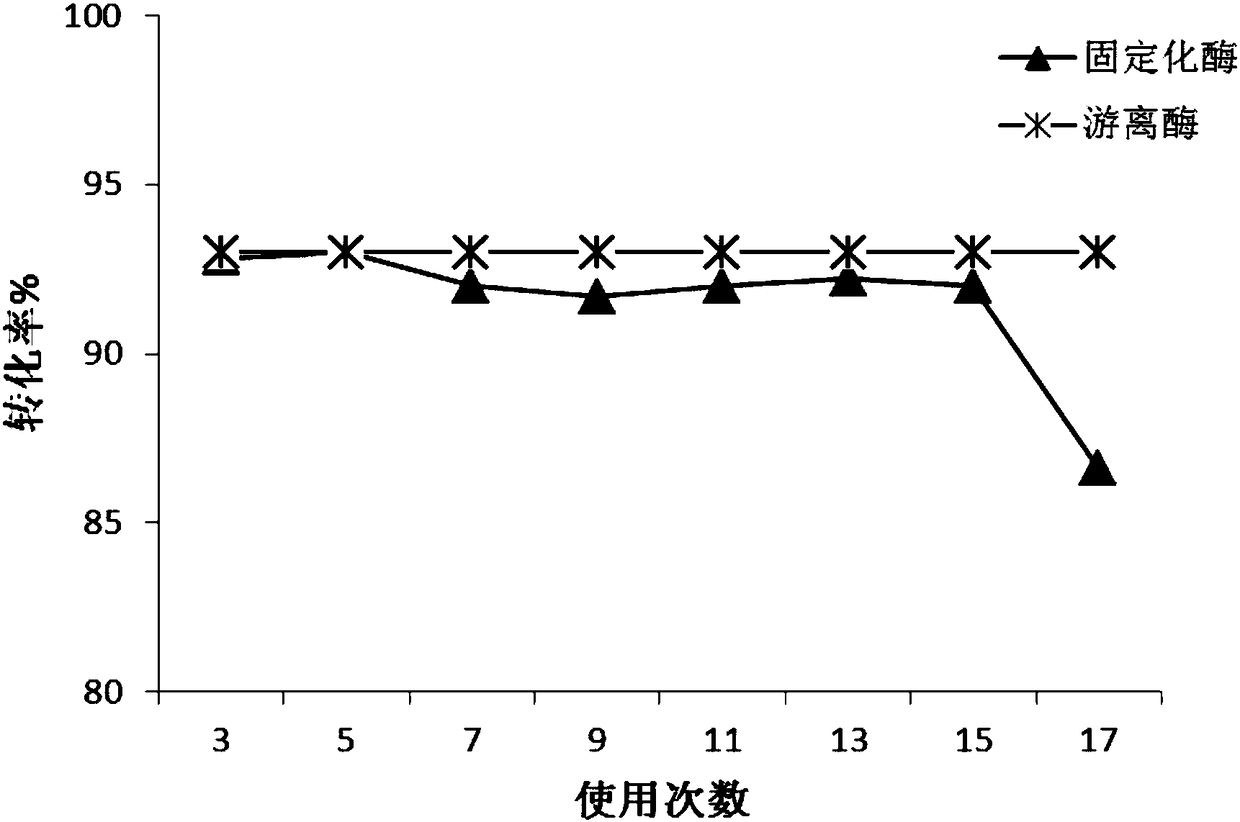
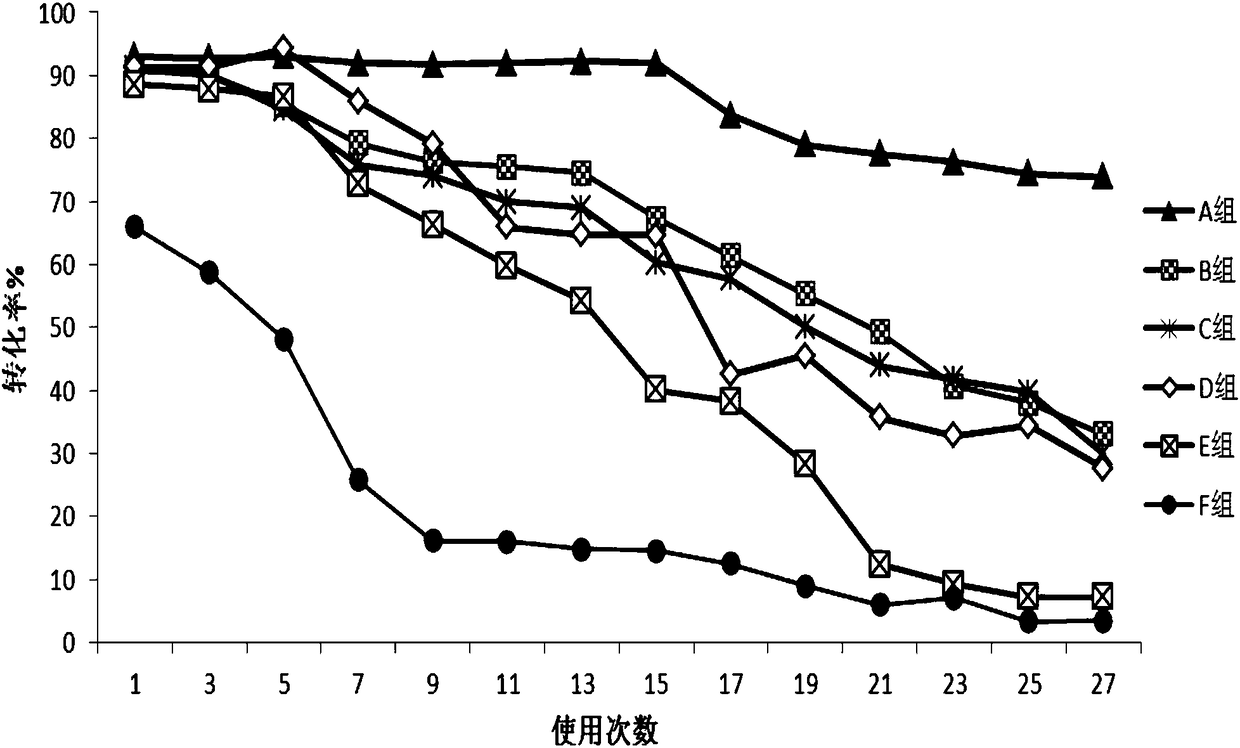
[0056] Example 1 Preparation of Epoxy Carrier Covalently Immobilized Trypsin
[0057] Get 2g (ES-102, Tianjin Nankai Hecheng Science and Technology Co., Ltd., http: / / www.tjhecheng.com / Products.aspx?id=84 of average particle diameter 450 μ m, average pore diameter is the epoxy-based carrier 20 times of enzyme molecule size ), washed 5 times with 1M potassium phosphate buffer (K 2 HPO 4 / KH 2 PO 4 , pH 7) washed once, and then drained for later use. Then take 100mg of trypsin dry powder (Shanghai Linye Biotechnology Co., Ltd., batch number 20140306), dissolve it in 10mL of 1M potassium phosphate buffer solution with pH=7, then add 2g of the epoxy-based carrier that has been treated above, and place it in a 4°C freezer Shaking reaction at 150rpm in the medium for 16h, after the shaking reaction, continue to place it in a freezer at 4°C for 15h. After the standing reaction, the immobilized enzyme reaction solution was taken out from the 4°C freezer, the pH value of the reacti...
Embodiment 2
[0059] Example 2 Preparation of Epoxy Carrier Covalently Immobilized Trypsin
[0060] Take 2 g of epoxy-based carriers (ES-101, Tianjin Nankai Hecheng Technology Co., Ltd.) with an average particle size of 300 μm and an average pore size of 20 times the size of enzyme molecules, wash with water for 5 times, and rinse with 2M potassium phosphate buffer (K 2 HPO 4 / KH 2 PO 4 , pH 8) washed once, and then drained for later use. Then take 100mg of trypsin dry powder (Shanghai Linye Biotechnology Co., Ltd., batch number 20140306), dissolve it in 20mL of 1M potassium phosphate buffer solution with pH 8, then add 2g of the epoxy-based carrier that has been treated above, and place it in a 4°C freezer at 150rpm The shaking reaction was performed for 28 hours, and after the shaking reaction was completed, it was placed in a freezer at 4°C for 25 hours. After the standing reaction, the immobilized enzyme reaction solution was taken out from the 4°C freezer, and the pH value of the r...
Embodiment 3
[0062] Example 3 Preparation of Amino Carrier Covalently Immobilized Trypsin
[0063] Take 2 g of an amine-based carrier (ReliZyme HA403 provided by Resindion S.R.L) with an average particle size of 200 μm and an average pore size 15 times the size of the enzyme molecule, and add it to glutaraldehyde (purchased from Xilong Chemical Industry, AR grade, concentration 50%)-phosphoric acid Activation was carried out in buffer solution (20mM, pH7), with a total volume of 10mL, in which the concentration of glutaraldehyde was 1% by mass, placed on a shaking table at 25°C and 150rpm for 2h, washed and dried for later use. Then take 100mg of trypsin dry powder (Shanghai Linye Biotechnology Co., Ltd., batch number 20140306), dissolve it in 10mL of 1M potassium phosphate buffer solution with pH 7, then add 2g of the activated amine-based carrier, and place it in a 4°C freezer at 150rpm The shaking reaction was performed for 16 hours, and after the shaking reaction was completed, it was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com