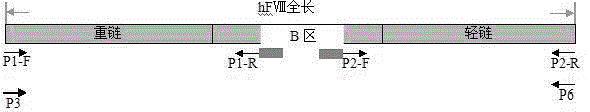
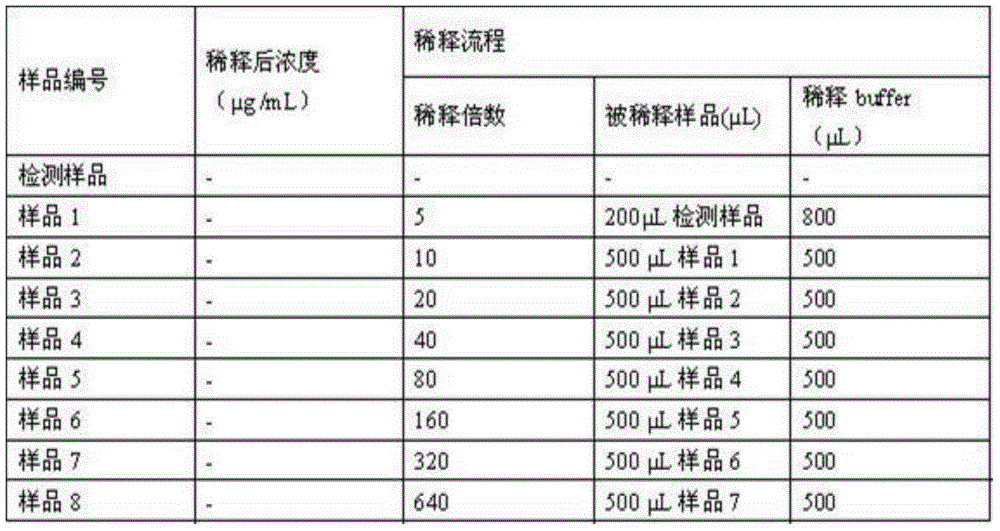
Zone B partially-deleted type recombinant human blood coagulation factor VIII
A technology of human coagulation factor and deletion type, applied in coagulation/fibrinolytic factor, factor VII, blood diseases, etc., can solve the problems of continuous shortage of FⅧ supply and insufficiency of rescue drugs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] The following examples are only illustrations of the present invention, and do not impose any limitation on the present invention.
[0026] (1) Acquisition and cultivation of production cell lines.
[0027] 1. CHO-DG44 cells.
[0028] Chinese hamster cells - the dihydrofolate reductase (dhfr)-deficient mutant DG44 is similar to the DXB11 mutant cell line used in the early 1980s. The cells require hypoxanthine (or adenine), glycine and thymine for growth, and like all CHO cells, they also require proline for growth. It has the advantage of not containing endogenous dhfr sequence, can be transformed by dhfr gene, and is convenient for screening of positive transformants. For cell lines transformed with the dhfr gene, dhfr-positive transformants are usually screened out with -MEM medium without nucleotides and deoxynucleotides. Since DG44 is a double-deletion mutant that does not contain the hamster dhfr gene, there is no background interference when the cell is introdu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com