Recombinant human growth hormone and its expression method in eukaryotic system
A technology of human growth hormone and nucleotide sequence, which is applied in the field of expression of recombinant human growth hormone and its eukaryotic system, can solve the problems of low expression, difficulty in industrialization, protein structure differences, etc., and achieve consistent biological activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
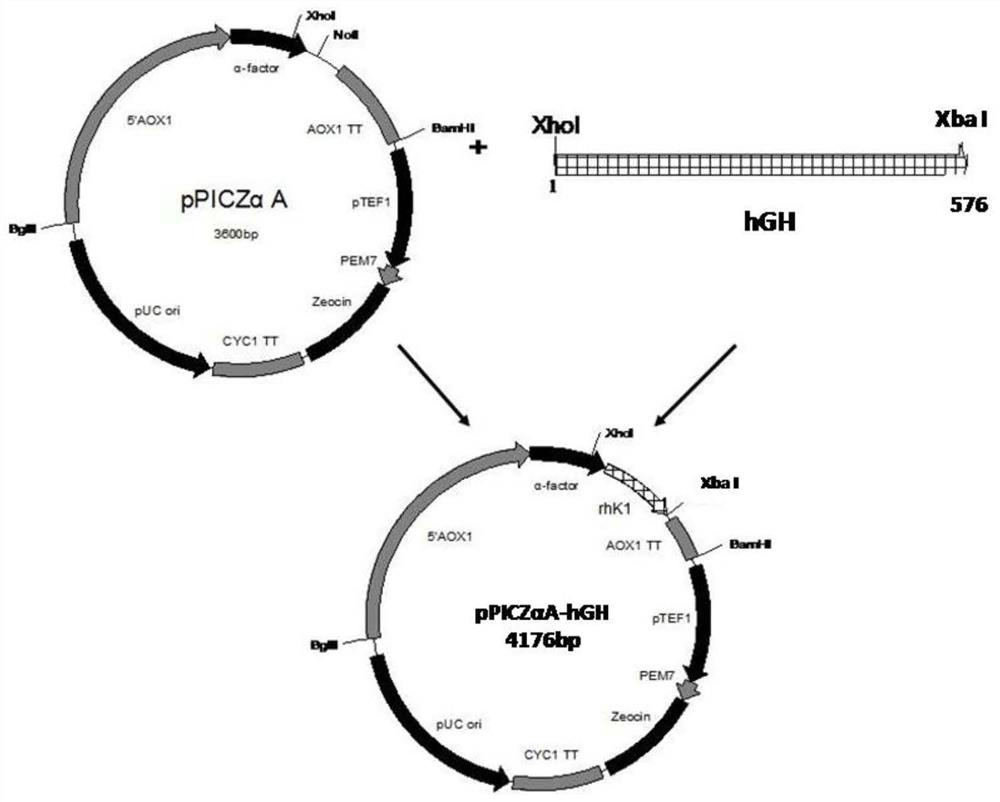
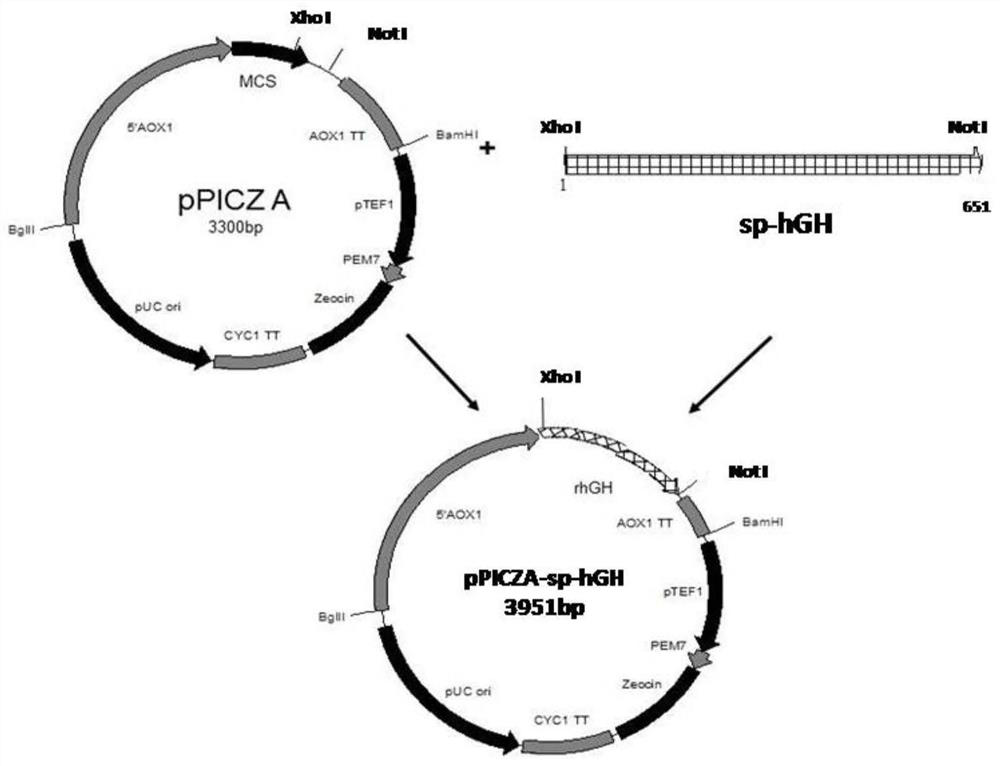
[0036] Embodiment 1: Construction of different recombinant hGH expression vectors
[0037] 1. Construction of recombinant hGH expression vector containing Saccharomyces cerevisiae α-factor signal peptide and short spacer peptide
[0038] According to the hGH cDNA sequence published by GenBank (GenBank accession number: E00141), the inventor optimized the codon of the gene to obtain the gene of the present invention without hGH self-signal peptide, and the nucleotide sequence is shown in SEQ ID NO.5 , the amino acid sequence is shown in SEQ ID NO.6, and constructed into the pUC57 plasmid (provided by Nanjing GenScript Co., Ltd.), to obtain a long-term preservation plasmid, which is denoted as pUC57-hGH plasmid.
[0039] Using the pUC57-hGH plasmid as a template, different spacer short peptides EA were inserted behind the Xho I restriction site for PCR amplification. The primers used were as follows:
[0040] Recombinant hGH upstream amplification primer (SEQ ID NO.9) containin...
Embodiment 2
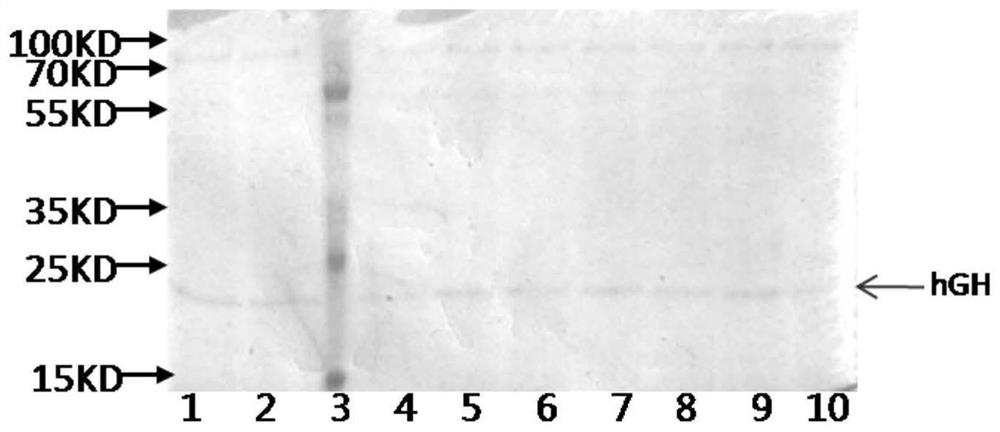
[0060] Example 2: Induced expression of different recombinant hGH engineering strains
[0061] 2.1 Screening of recombinant hGH host engineering strains:
[0062] The X-33 Pichia strain was prepared into electrocompetent cells according to the instructions of the Invitrogen Company's Easy SelectPichia Expression Kit. The six plasmids obtained in Example 1 were digested and linearized with Sac I restriction endonuclease (R0156S, purchased from New England Biolabs), and after ethanol precipitation, the linearized vector was electrotransformed into Pichia pastoris competent cells , spread on YPDS solid medium, and culture at 30°C until the transformants grow out.
[0063] Preparation of YPDS solid medium: Invitrogen Company Easy SelectPichia Expression Kit instructions provided, in which yeast extract 10g / L, peptone 20g / L, glucose 20g / L, agarose 15g / L, 182g / L.
[0064] 2.2 Methanol-induced expression of recombinant hGH engineering strains
[0065] Pick the host monoclonal en...
Embodiment 3
[0070] Embodiment 3: Purification of hGH recombinant protein
[0071] This patent mainly uses a two-step method to purify hGH protein. The first step uses cationic chromatography to enrich hGH and remove pigment, and the second step uses hydrophobic exchange chromatography to remove other foreign proteins. Specific steps are as follows:
[0072] 1. Pretreatment of fermentation broth
[0073] The shake flask supernatant obtained by the method in Example 2 was diluted with pH 4.5, 50 mM NaAc buffer solution to conductance <5 mS / cm, and filtered through a 0.45 μm filter membrane to obtain a purified sample after treatment, which can be purified by cationic chromatography.
[0074] 2. Purification by Cation Chromatography
[0075] Put the above treated purified sample on a 5ml Hitrap SP HP prepacked column, the equilibration buffer is 50mM NaAc, pH 4.5, the elution buffer is 50mM NaAc, 1.0M NaCl, pH 4.5, and wash according to 0-100% linear The hGH protein is mainly concentrat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com