A kind of preparation method of high-purity recombinant human brain natriuretic peptide
A technology of human brain natriuretic peptide and fusion protein is applied in the field of preparation of fusion tag protein and high-purity recombinant human brain natriuretic peptide. Expression, avoid renaturation, reduce the effect of production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
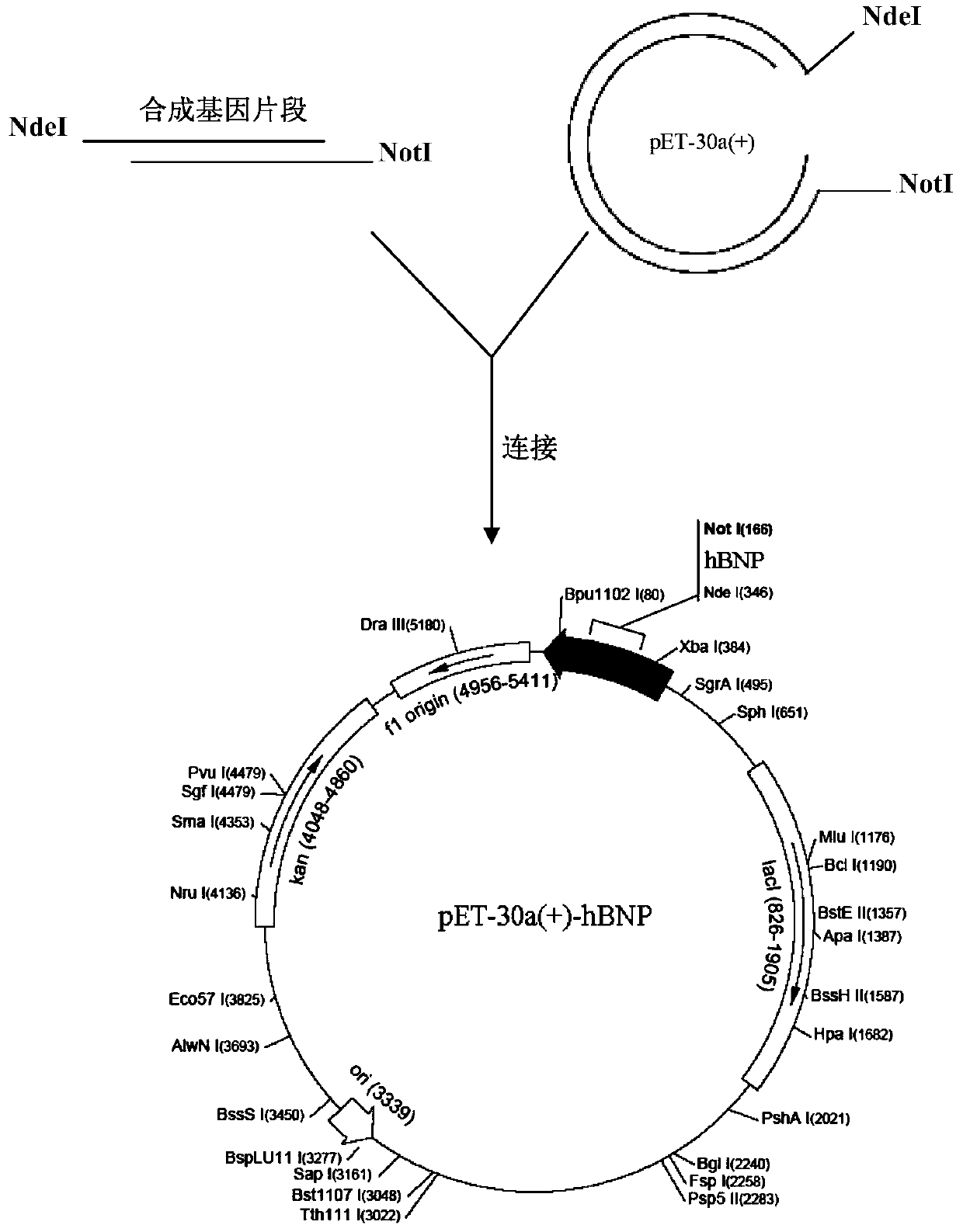
[0033] Example 1 Construction of recombinant human brain natriuretic peptide fusion protein engineering bacteria
[0034] 1. Construction of pET30-a(+)-hBNP / BL21(DE3) engineering bacteria
[0035] The human brain natriuretic peptide mature protein sequence (GenBank accession number: NP_002512.1) is 32 amino acids, and the human brain natriuretic peptide gene sequence is shown in SEQ ID NO.1.
[0036] The tag protein is selected from the complete sequence of Escherichia coli MrsB (GenBank accession number: BAA15575.2); the amino acid sequence of the fusion tag protein is shown in SEQ ID NO.2, and its nucleotide sequence is shown in SEQ ID NO.3 .
[0037] The street sequence was designed as -HHHHHHGGSDDDDK-, and the corresponding nucleic acid sequence was CACCATCATCATCATCATGGTGGTTCTGACGACGACGACAAG as a linker, and the C-terminal of the above-mentioned MrsB tag protein was connected to the N-terminal of the human brain natriuretic peptide mature protein sequence through the link...
Embodiment 2
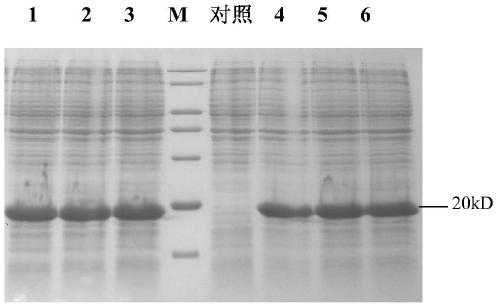
[0043] Embodiment 2 Fermentation of recombinant human brain natriuretic peptide engineering bacteria
[0044] Streak LB plates (kan 100mg / L) with pET30-a(+)-hBNP / BL21(DE3) engineered bacteria and culture them in a constant temperature incubator at 37°C for about 16-18h until a single colony grows. A single colony of engineered bacteria was picked and inoculated into 20ml LB medium (kan 100mg / L), and cultured at 37°C and 230rpm for 8h. 0.1% was transferred to 250ml LB (kan 100mg / L), 1L conical flask, cultured at 37°C, 230rpm for 13h. Four bottles were cultured in parallel, 1000ml of bacterial solution was prepared, and 5% was inoculated into the fermenter NLF-2220L fermentation medium (TB medium). Before inoculation, the pH was adjusted to 7.0 with ammonia water, and the temperature during the fermentation process was controlled at 36°C. The pH value and dissolved oxygen of the fermentation medium are controlled by adding ammonia water and increasing the stirring speed and ven...
Embodiment 3
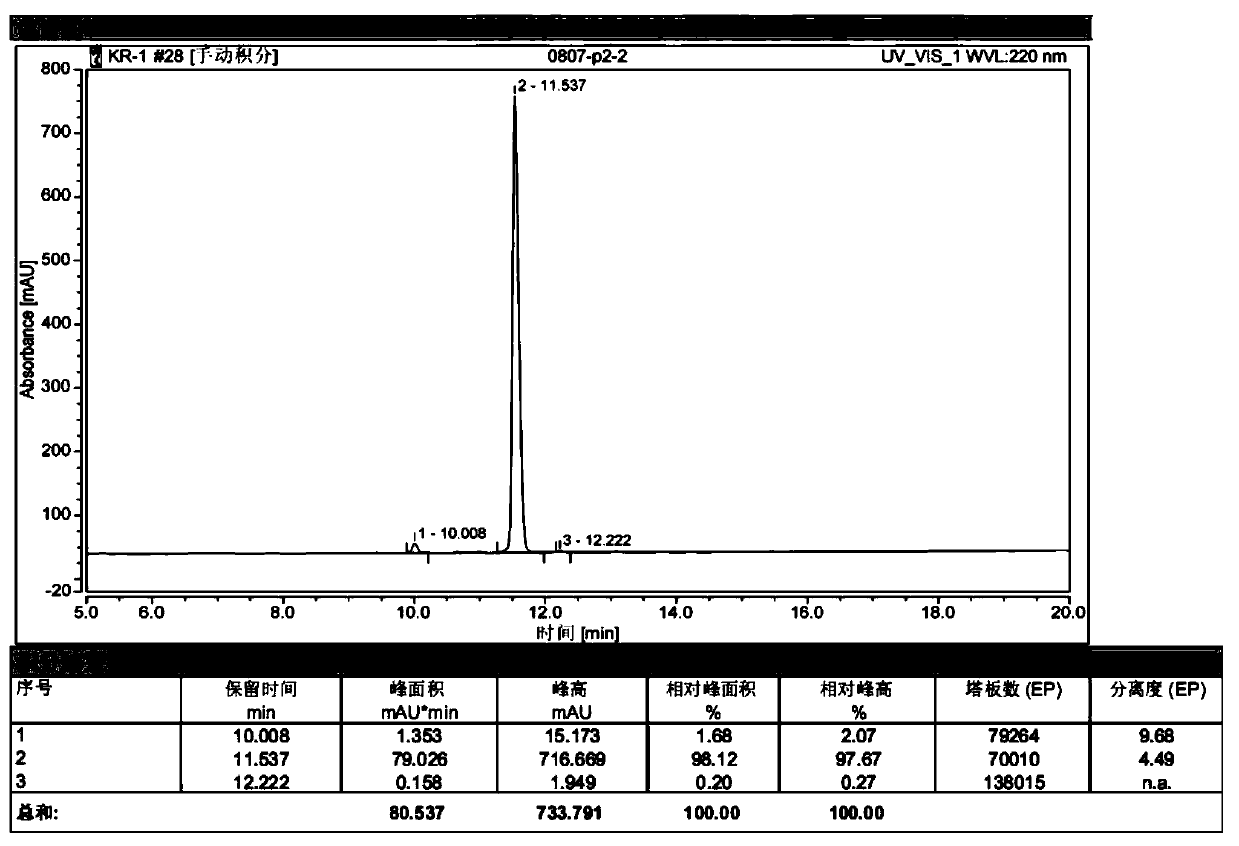
[0046] Example 3 Separation and Purification of Recombinant Human Brain Natriuretic Peptide
[0047] The bacteriostasis supernatant was loaded on Ni2+-Chelating Sepharose Fast Flow (GE Healthcare) chelation chromatography medium treated with 0.2M NiSO4 and equilibrated with 20mM Tris-HCl (pH8.0), and 200mM imidazole (containing 20mM Tris- HCl, pH 8.0) elution; the purpose fusion protein collected was desalted, and 0.5U enterokinase (50mM Tris-HCl, 2mM CaCl , 0.1% Tween-20, pH 8.0) was added to each 1mg fusion protein at 6- Digest the fusion protein for about 17 hours at 8°C.
[0048] The digested target protein is loaded on Ni2+-Chelating Sepharose Fast Flow, and the target protein and fusion tag are simultaneously hung on the column. However, the binding capacity of the target protein on the column was low, and the target protein was eluted with 50 mM concentration of imidazole (Shanghai Sangong, batch number: B421BA0025), and the peak of the eluted protein was collected. A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com