Recombinant histolytic clostridium type II collagenase, preparation method and application thereof
A technology of Clostridium histolyticum and collagenase, which is applied in the field of bioengineering, can solve the problems of complex refolding process, high technical difficulty, and high production cost, and achieve the effects of purification, soluble expression, and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
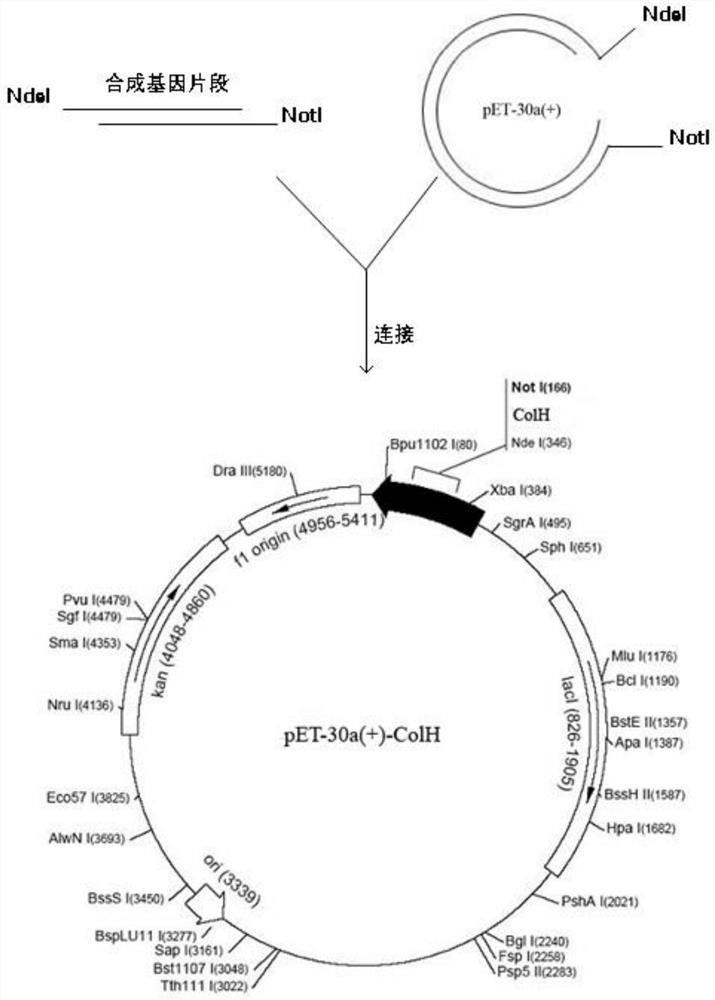
[0045] Example 1 Construction of Recombinant Clostridium histolytica Type II Collagenase Fusion Protein Engineering Bacteria
[0046] 1. Construction of pET30-a(+)-ColH / BL21(DE3) engineering bacteria
[0047] The mature protein of Clostridium histolyticum type II collagenase contains 991 amino acids, and the sequence of the Clostridium histolyticum type II collagenase gene is shown in SEQ ID NO.1.
[0048] The tag protein is selected from Fasciola hepatica calcium-binding protein Fh8 (GenBank accession number: AAF31420.1); the amino acid sequence of the tag protein protein is shown in SEQ ID NO.2, and its nucleotide sequence is shown in SEQ ID NO. 3.
[0049] The linker sequence was designed as SEQ ID NO: 7-GSGSGHMHHHHHHSSGPDLDDDDK-, and the C-terminus of the above-mentioned Fh8 tagged protein was connected to the N-terminus of the mature protein sequence of Clostridium histolyticum type II collagenase through the linker. The amino acid sequence of the obtained fusion protein...
Embodiment 2
[0055] Example 2 Fermentation of Recombinant Clostridium histolyticum Type II Collagenase Engineering Bacteria
[0056] Seed liquid activation: transfer 60ul of pET30-a(+)-ColH / BL21(DE3) glycerol tube strain into 100ml 2xYT medium, kan+100ug / ml; culture temperature 33℃, 220r / min, culture for about 12h (OD value 4.5-5.5).
[0057] Fermentation process: use 5L Baoxing fermenter for fermentation, set the temperature at 37°C, ventilate at 3vvm, insert 4ml of trace elements, kan+0.06g sterile filter, then insert into the fermenter pH7.0; take LB culture medium stored at 4°C The prepared seeds are inoculated into 2L of fermentation medium (M9 improved medium) according to the proportion of 5% (100ml); the initial speed is 300rpm, start the speed dissolved oxygen linkage, the dissolved oxygen value is set to 30%, and the speed is increased to 600r / min, the dissolved oxygen value is set to 15%, and the speed is increased to 800r / min. At this time, the ventilation volume is adjusted ...
Embodiment 3
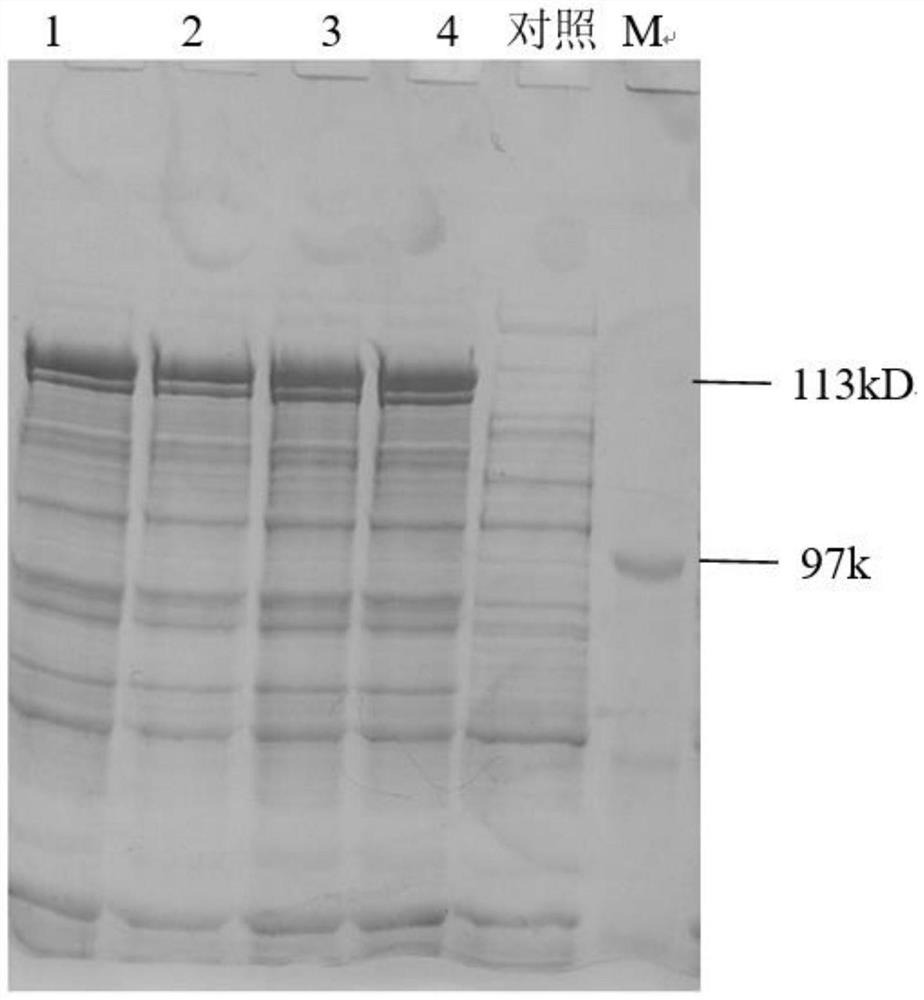
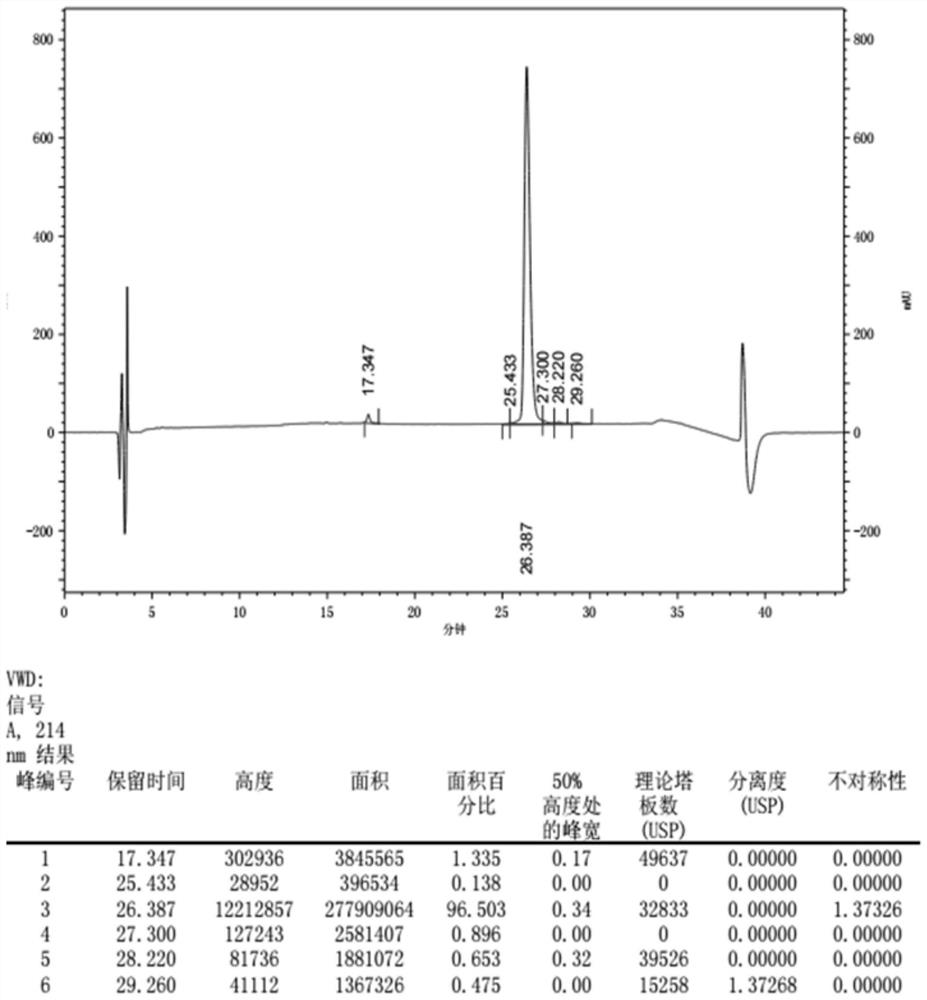
[0058] Example 3 Separation and Purification of Recombinant Clostridium Histolyticum Type II Collagenase
[0059] 1. Bacterial crushing and clarification
[0060] The bacteria were collected by high-speed freezing and centrifugation, and the bacteria were suspended according to the ratio of 1:10 (1g:10ml buffer: 20mM Tris-HClpH7.6). Break the bacteria 3 times, then refrigerate and centrifuge at high speed, collect the supernatant, and discard the precipitate; use a 0.65 μm hollow fiber column to clarify the bacteriostasis solution by ultrafiltration.
[0061] 2. Ni 2+ Chelation chromatography
[0062] Use equilibration buffer 25mM Tris-HCl pH8.0, equilibrate the chromatography column, load the bacteriostasis solution, then use elution buffer 25mM Tris-HCl pH8.0 10mM imidazole to elute impurity protein; use elution buffer 25mM Tris-HCl pH8 .0 50mM imidazole to elute the fusion protein of interest.
[0063] 3. Enterokinase enzymolysis
[0064] In the above fusion protein so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com