A method for increasing the expression level of soluble recombinant prokinein 2β in Escherichia coli
A protokinin, Escherichia coli technology, applied in the field of genetic engineering biology, can solve the problems of complex process, cumbersome operation, and high purification cost, and achieve the effects of improving solubility, simple and easy process operation, and improving soluble expression.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
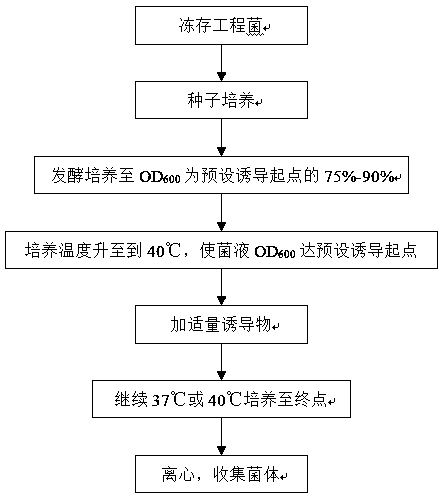
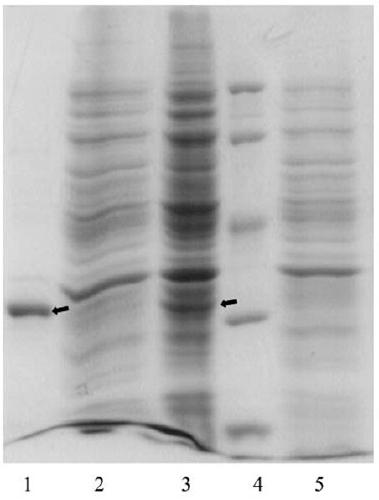
[0022] Demo strain used E.coli BL21(DE3)pET-PK2β (Wen Chongwei et al. Study on optimization of induction conditions for recombinant protokinesin 2β by response surface analysis. Biotechnology Bulletin. 2012. Pages 173-178) The engineering bacteria are self-constructed. The LB liquid medium used contained 10 g of peptone, 5 g of yeast extract, and 10 g of sodium chloride per liter. The prepared LB medium was sterilized in an autoclave at 121°C for 20 minutes. Then, kanamycin was added to the sterilized LB medium to a final concentration of 50 μg / mL to obtain the fresh LB medium for expression used in the examples. At the same time, lactose is used as the inducer, and lactose is added to the final concentration of 1g / L in the examples. EDTA broken bacteria liquid contains 0.05 mol•L -1 Tris•HCl pH 8.0, 0.10 mol•L -1 NaCl, 0.025mol•L -1 EDTA; hypertonic liquid is 0.02 mol•L -1 Tris•HCl, 0.025 mol•L -1 EDTA, pH 8.0, 35% (w / v) sucrose, hypotonic solution is 0.02 mol•L -1 T...
Embodiment 2
[0029] Demo strain used E.coli BL21 (DE3) pET-PK2β (Wen Chongwei et al. Study on optimization of induction conditions for recombinant protokinesin 2β by response surface analysis. Biotechnology Bulletin. 2012. Pages 173-178) The engineering bacteria are self-constructed. The LB liquid medium used contained 10 g of peptone, 5 g of yeast extract, and 10 g of sodium chloride per liter. The prepared LB medium was sterilized in an autoclave at 121°C for 20 minutes. Then, kanamycin was added to the sterilized LB medium to a final concentration of 50 μg / mL to obtain the fresh LB medium for expression used in the examples. At the same time, lactose is used as an inducer, and lactose is added to the final concentration of 1 g / L in the examples. EDTA broken bacteria liquid contains 0.05 mol•L -1 Tris•HCl pH 8.0, 0.10 mol•L -1 NaCl, 0.025mol•L -1 EDTA; hypertonic liquid is 0.02 mol•L for hypotonic liquid -1 Tris•HCl, 0.025 mol•L -1 EDTA, pH 8.0, 35% (w / v) sucrose, hypotonic solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com