Recombinant clostridium histolyticum type I collagenase as well as preparation method and application thereof
A technology of Clostridium histolyticum and collagenase, which is applied in the field of bioengineering, can solve the problems of complex renaturation process, high technical difficulty, and high production cost, and achieve the effects of facilitating purification, improving purification efficiency, and avoiding renaturation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
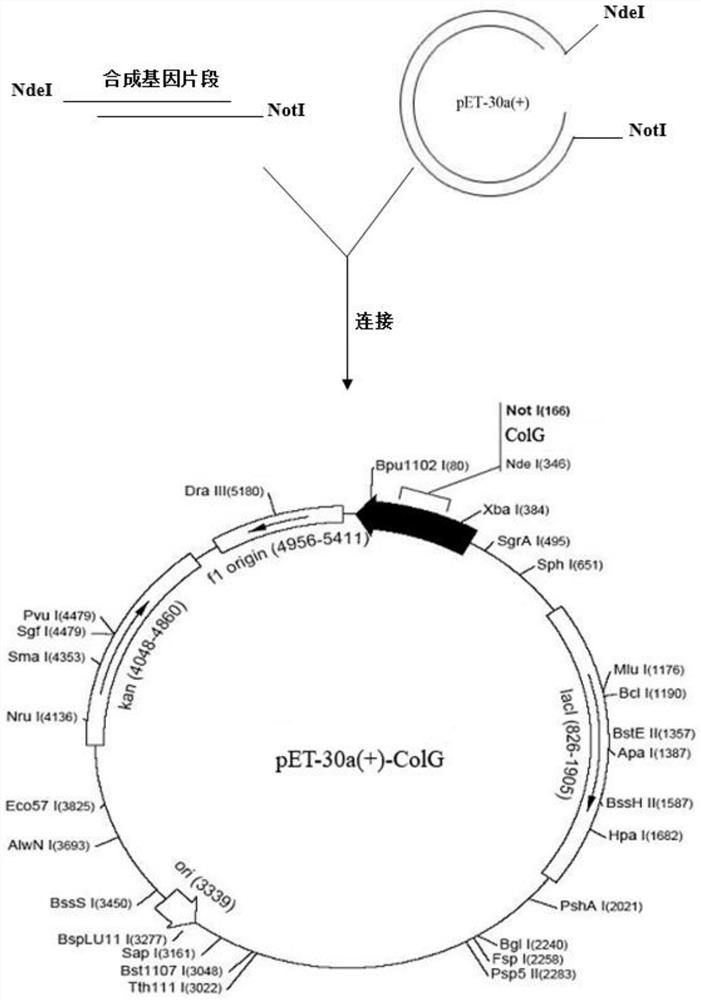
[0039] Example 1 Recombinant Clostridium histolytica Type I Collagenase Fusion Protein Engineering Bacteria Construction
[0040] 1. Construction of pET30-a(+)-ColG / BL21(DE3) engineering bacteria
[0041] The mature protein of Clostridium histolyticum type I collagenase contains 1008 amino acids, and the gene sequence of Clostridium histolyticum type I collagenase is shown in SEQ ID NO:2.
[0042] The tag protein is selected from the tandem combination of calmodulin-binding peptide and streptavidin-binding peptide; the amino acid sequence of the fusion tag protein is shown in SEQ ID NO: 3, and its nucleotide sequence is shown in SEQ ID NO: 4.
[0043] The linker sequence was designed as -GSGSGGTAMADIGSDDDDK-, and the C-terminus of the above-mentioned CBP-SBP tagged protein was connected to the N-terminus of the mature protein sequence of Clostridium histolyticum type I collagen through the linker. The amino acid sequence of the obtained fusion protein is shown in SEQ ID NO:9 ...
Embodiment 2
[0049] Embodiment 2 Recombinant Clostridium histolyticum Type I Collagenase Engineering Bacteria Fermentation
[0050] Seed liquid activation: divide the liquid LB medium into 500ml shake flasks, 100ml per bottle, kan+160ul, a total of 1 bottle, then absorb 40ul of glycerol seeds, inoculate in LB liquid medium on a shaker overnight at 27°C and 180rpm to cultivate.
[0051] Fermentation process: use 5L Baoxing fermenter for fermentation, set the temperature at 37°C, ventilate at 1.5vvm, add 4ml of trace elements, kan+0.06g sterile filter, then put into the fermenter pH7.0; take LB culture medium stored at 4°C The seeds prepared in the following steps are inoculated into 2L of fermentation medium (M9 improved medium) according to the proportion of 5% (100ml); the initial speed is 200rpm, and the dissolved oxygen speed is set to be linked, and the dissolved oxygen is not less than 30% (the equipment has 3-5% error, need to set the parameter 35%) to correct dissolved oxygen 100%;...
Embodiment 3
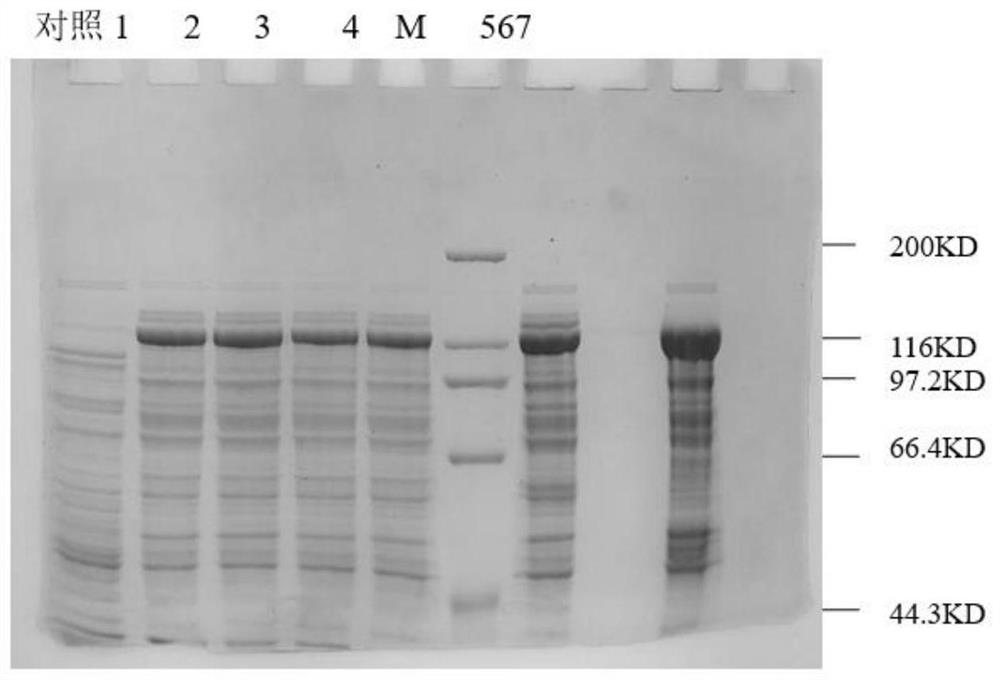
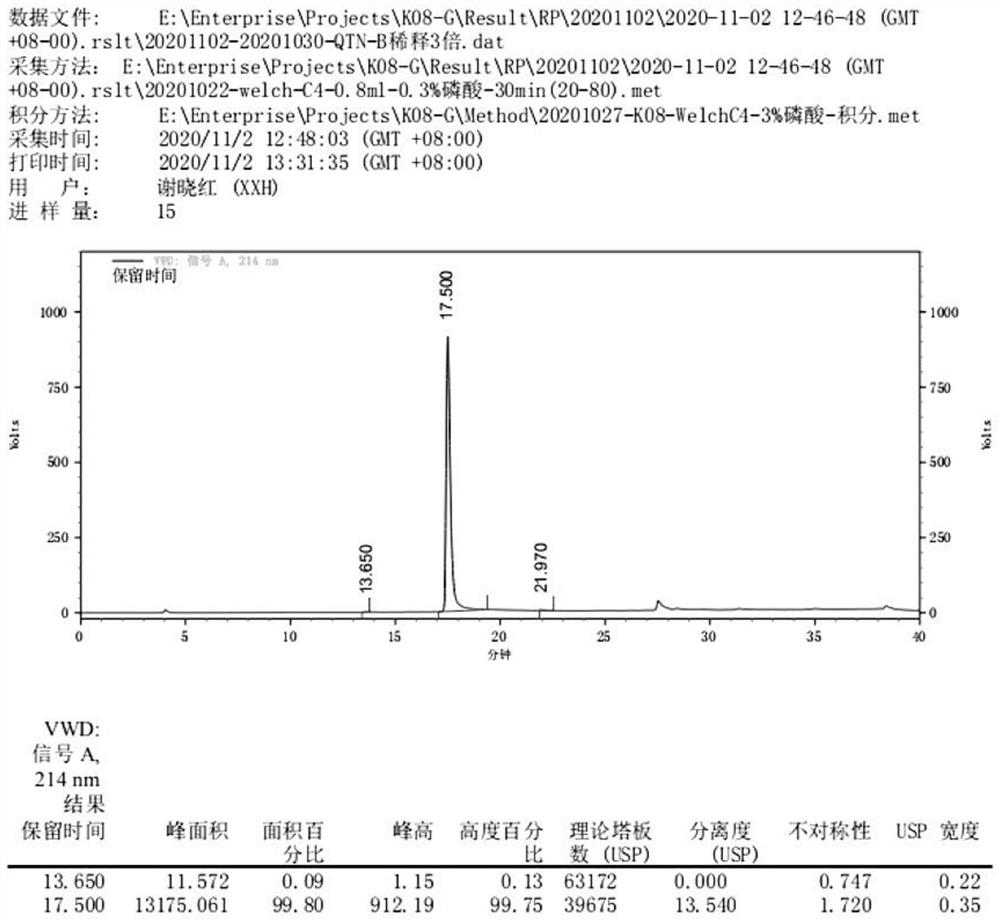
[0052] Example 3 Separation and Purification of Recombinant Clostridium histolyticum Type I Collagenase
[0053] 1. Bacterial crushing and clarification
[0054] The bacteria were collected by high-speed freezing and centrifugation, and the bacteria were suspended according to the ratio of 1:10 (1g:10ml buffer: 25mM Tris-HClpH8.0). Break the bacteria 3 times, then refrigerate and centrifuge at high speed, collect the supernatant, and discard the precipitate; use a 0.65 μm hollow fiber column to clarify the bacteriostasis solution by ultrafiltration.
[0055] 2. CBP affinity chromatography
[0056] Take the clarified supernatant and dilute it 2 times with low concentration calcium buffer. The protein of interest was captured with CalmodulinSepharose 4B affinity filler. Elution was performed with 2 mM EGTA (ethylene glycol diethyl ether diamine tetraacetic acid), pH 7.0.
[0057] 3. Enterokinase enzymolysis
[0058] In the above fusion protein solution, 0.5 U of recombinant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com