Method for preparing recombinant human blood coagulation factor VIII
A human blood coagulation factor and bioreactor technology, applied in the field of bioengineering, can solve the problems of high technical barriers in cell culture technology, difficulty in maintaining the state of cells, and serious cell clumping, so as to improve production, protein activity, cell viability and Increased protein expression and high mixing efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
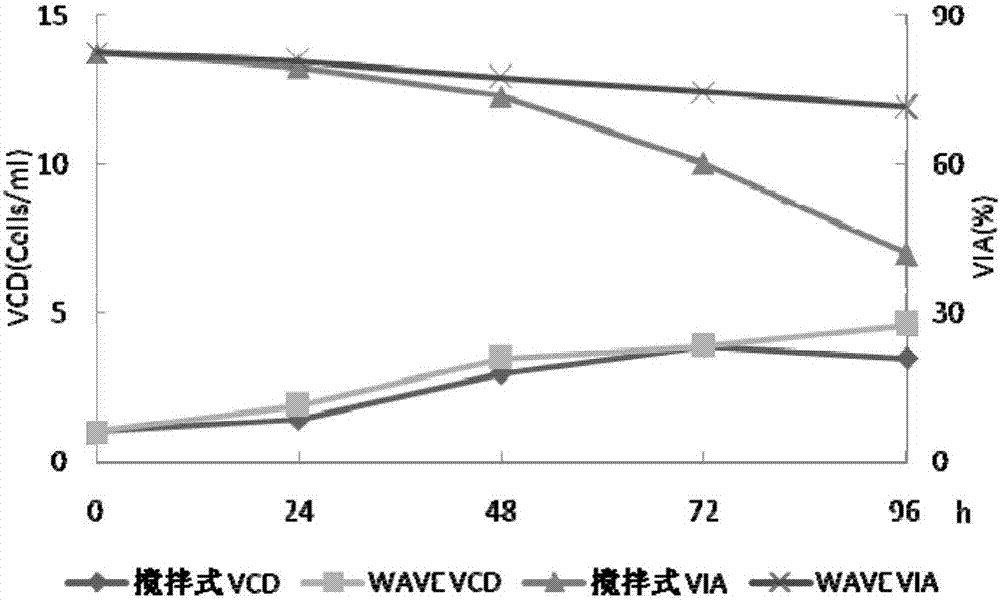
[0032] Example 1 Investigating the cell culture effect of WAVE wave bioreactor and stirred stainless steel reactor
[0033] First-level seed solution preparation: take a frozen recombinant human coagulation factor VIII working cell bank cell (self-made, 1ml) from the liquid nitrogen tank, thaw it in a water bath at 37°C, and transfer it to CD OptiCHO AGT containing 20ml seed medium ( In a 125ml cell culture shake flask containing 4mmol / L glutamine (Life technologies company), 50μg / ml zeocin (Invotrogen company, Life technologies company), placed in 37 ℃, 6~10%CO 2 Cultivate in a carbon dioxide constant temperature incubator at 110-130rpm. Observe the cell state every day, take samples for cell count and detect cell viability (trypan blue method), the cell density is about 3.0~4.0×10 6 Subculture at cells / ml, the subculture density is about 0.6~1.0×10 6 cells / ml, after 3 to 4 passages, inoculate the primary seed solution in the WAVE wave reactor (GE Healthcare, model 20 / 50EHT...
Embodiment 2
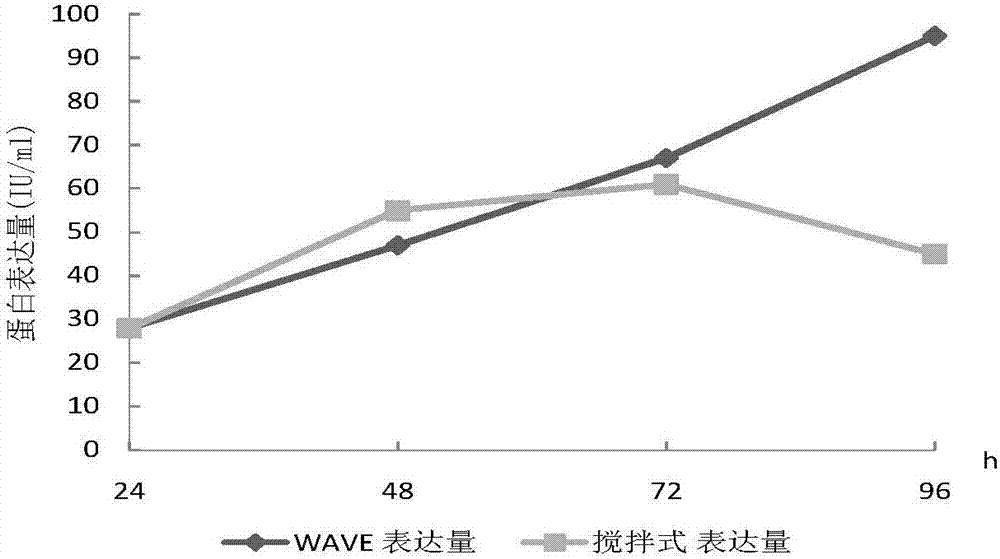
[0041] Example 2 WAVE wave bioreactor to investigate the effect of different culture periods on cell viability and protein expression
[0042] Refer to the preparation method of Example 1 to obtain the secondary seed liquid.
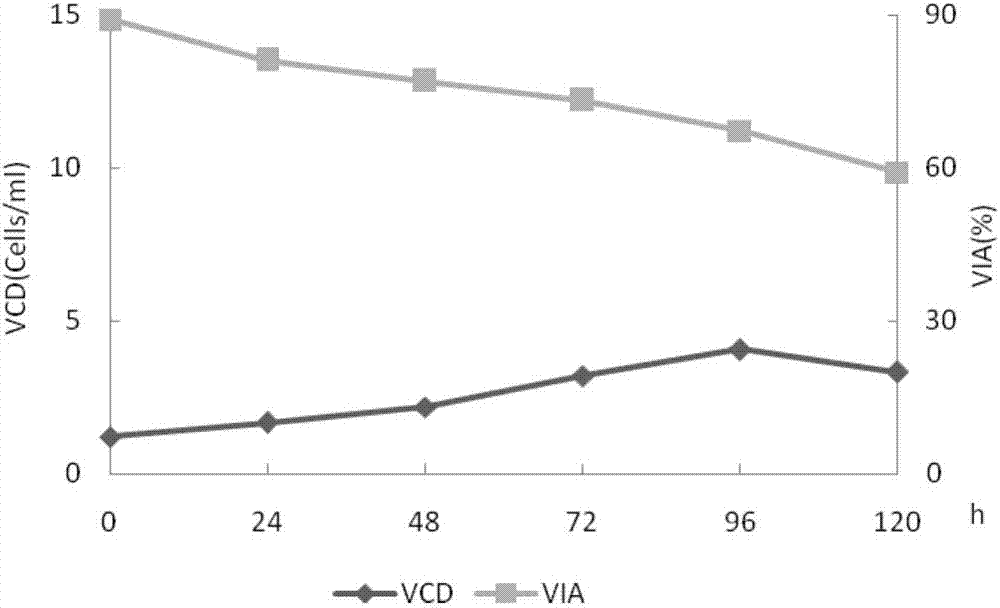
[0043] Inoculate the secondary seed solution at a ratio of 1 / 4 to 1 / 3, 0.6 to 1.0×10 6 The seeding density of cells / ml, the medium is CDOptiCHO AGT (containing 4mmol / L glutamine (Life technologies company), 0.02% antifoam C (SIGMA company), 1g / L Kolliphor P188 (sigma company), Life technologies company), For fed-batch culture, add 200mmol / L glutamine to a concentration of 2-4mmol / L at the 24th to 72nd hour of the culture cycle, and add 200g / L glucose (Sigma Company) solution until the glucose content is 2-4g / L. L, harvested at the 120th hour of the culture cycle. During the culture process, samples were taken every 24 hours for cell counting to detect cell viability (trypan blue method) and protein activity (one-phase method). WAVE wave reactor cell c...
Embodiment 3
[0045] Example 3 Purification of recombinant human blood coagulation factor VIII
[0046] (1) Harvest the cell culture medium
[0047] In the cell suspension (WAVE wave reactor cultivation) that embodiment 1 obtains, add the damping fluid (10mmol / L Hepes, 5mmol / L calcium chloride dihydrate, 4mol / L chloride) containing sodium chloride and calcium chloride Sodium, pH7.2), so that the final concentration of sodium chloride is about 0.5mol / L, so that the conductivity at 2~8°C is 40~50mS / cm, mix the solution and let it stand for about 30 minutes, and filter it through deep layer Cell removal was followed by sterile filtration (0.22 μm) to remove any remaining cell debris and particulate matter.
[0048] (2) S / D virus inactivation
[0049] Virus inactivation was performed on the clarified cell harvest liquid obtained in step (1) using 0.3% tributyl phosphate (TNBP) (v / v) and 1% Triton X-100. Stir at 20-25°C for 45 minutes to inactivate the virus.
[0050] (3) Affinity chromatogr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com