Method for preparing cryoprecipitate and method for preparing blood coagulation factor VIII preparation by using cryoprecipitate
A technology of cryoprecipitation and polyethylene glycol precipitation, which is applied in the preparation method of peptides, coagulation/fibrinolytic factors, factor VII, etc., can solve the problems of waste of plasma resources and less cryoprecipitation, and achieve full utilization and good economic benefits , the effect of good market application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
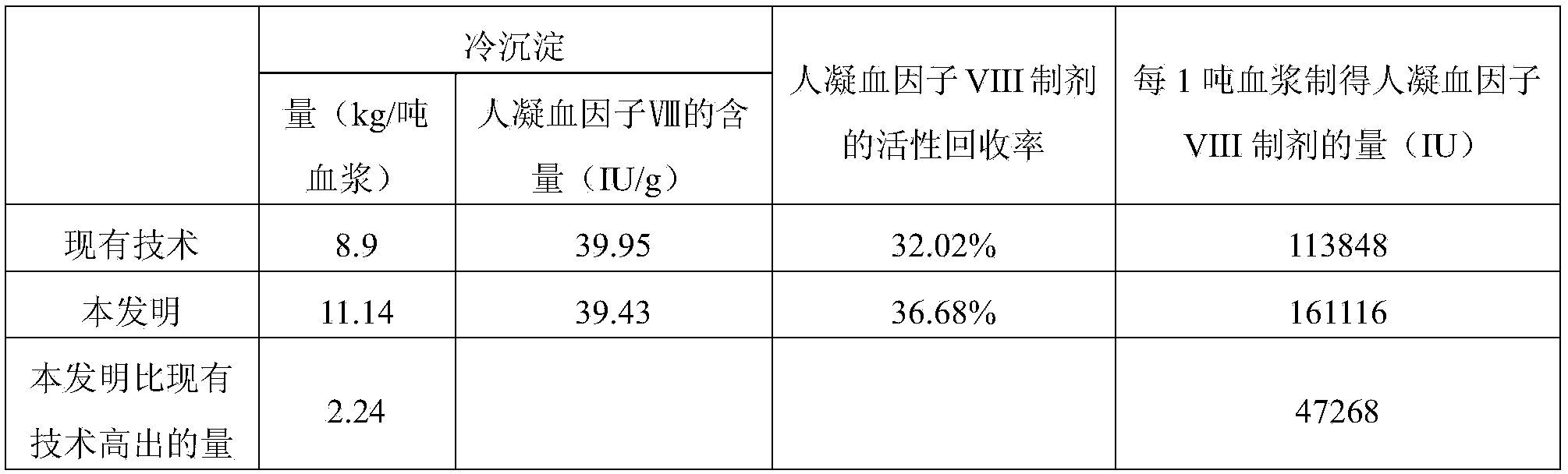
[0032] Embodiment 1 Prepare cryoprecipitate with the method of the present invention
[0033] 1. Experimental equipment
[0034] Weir filter: model is FL-2023 weir tank filter;
[0035] Continuous centrifuge: GQ142 high-speed tube centrifuge.
[0036] 2. Experimental method
[0037] (1) After collection of fresh healthy human plasma, store it at -20°C for no more than 3 years;
[0038] (2) Pre-thawing: put 2500L of fresh frozen plasma (2575Kg, the content of human coagulation factor VIII is 2500,000IU), place it at an ambient temperature of 0°C, and heat it up to -10°C to 0°C;
[0039] (3) Melting: melt in a water bath at 25°C, and heat the plasma to 0-5°C to melt the plasma;
[0040] (4) Filtration: Under the condition of maintaining the temperature of the melted plasma at 0-5°C, filter with a weir filter to obtain filtrate and filter residue;
[0041] (5) Centrifugation: Under the condition of maintaining the temperature of the filtrate at 0-5°C, use a continuous centri...
Embodiment 2
[0047] Embodiment 2 Prepare cryoprecipitate with the method of the present invention
[0048] 1. Experimental equipment
[0049] Weir filter: model is FL-2023 weir tank filter;
[0050] Continuous centrifuge: GQ142 high-speed tube centrifuge.
[0051] 2. Experimental method
[0052] (1) After collection of fresh healthy human plasma, store it at -20°C for no more than 3 years;
[0053] (2) Pre-thawing: put 2500L of fresh frozen plasma (2575Kg, the content of human blood coagulation factor VIII is 2500,000IU), place it at an ambient temperature of 2°C, and heat it up to -10°C to 0°C;
[0054] (3) Melting: melt in a water bath at 37°C, and heat the plasma to 0-5°C to melt the plasma;
[0055] (4) Filtration: Under the condition of maintaining the temperature of the melted plasma at 0-5°C, filter with a weir filter to obtain filtrate and filter residue;
[0056] (5) Centrifugation: Under the condition of maintaining the temperature of the filtrate at 0-5°C, use a continuous ...
Embodiment 3
[0058] Example 3 Preparation of human blood coagulation factor VIII preparation by cryoprecipitation of the present invention
[0059] 1. Experimental method
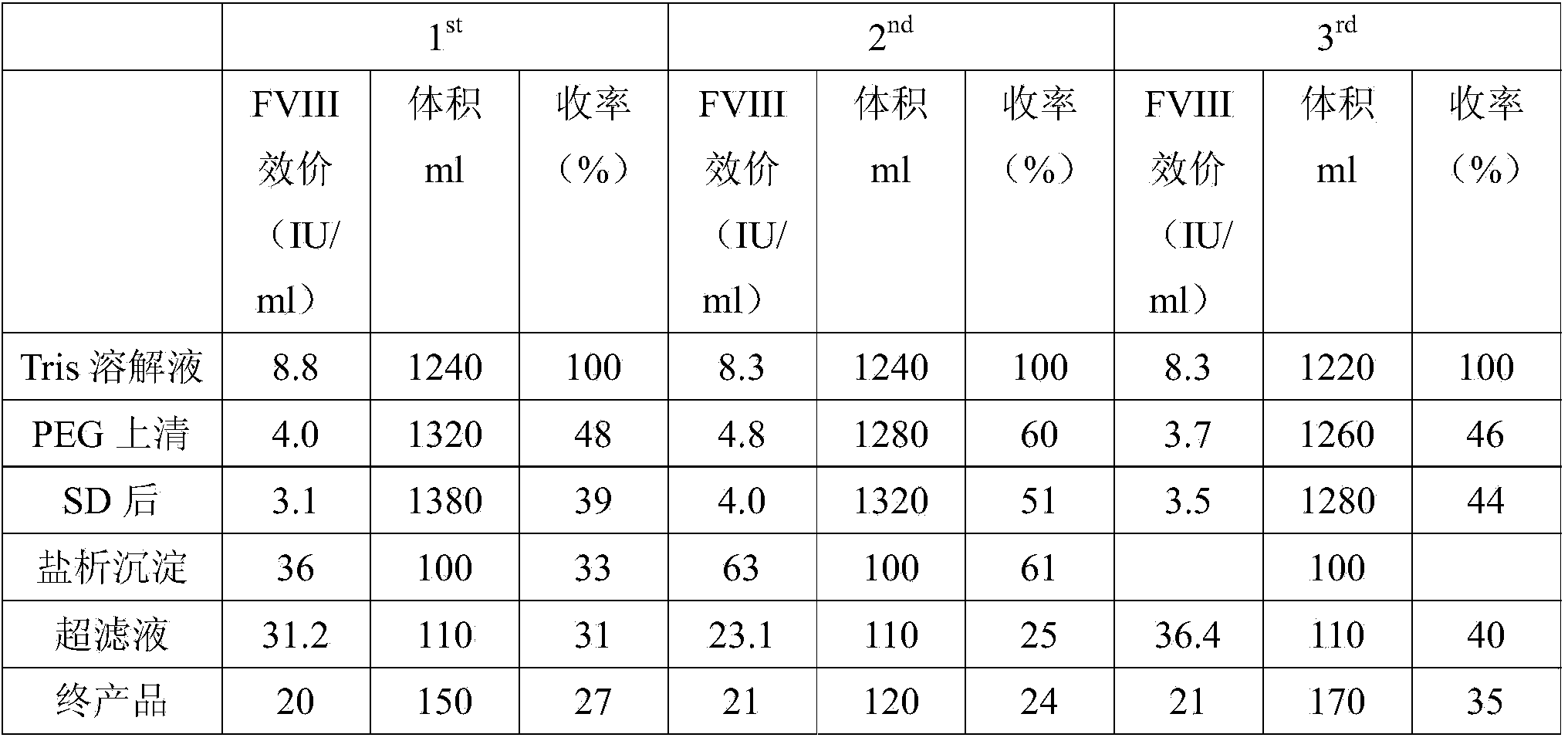
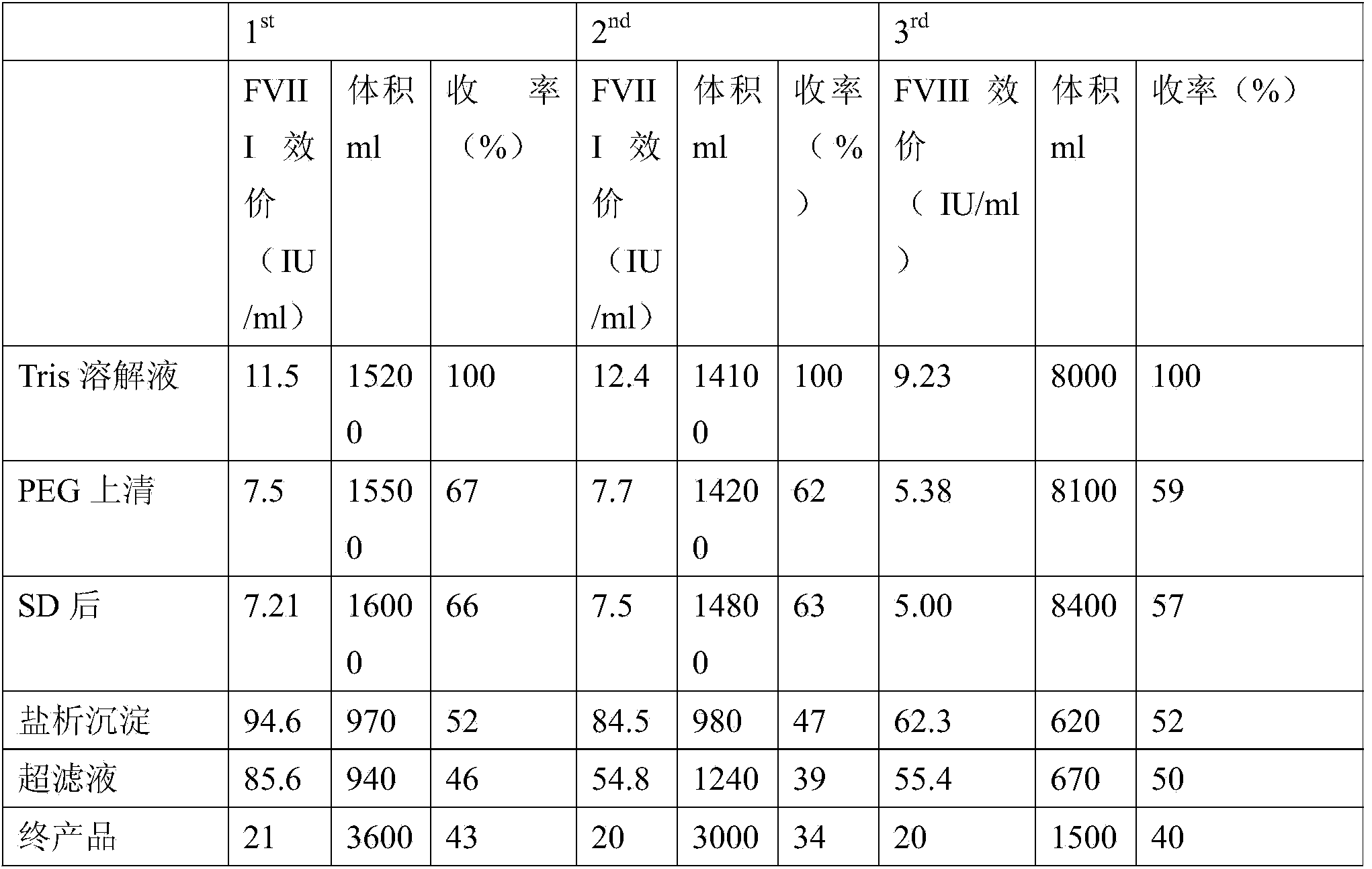
[0060] 300g of the cryoprecipitate (filter residue) obtained by filtration in step (4) of Example 1 (repeated three times) and the cryoprecipitate (precipitate) obtained by centrifugation in step (5) of Example 1 (the weights are 3.8kg, 3.525kg, 2kg respectively) according to The following method is used to purify and prepare human coagulation factor VIII:
[0061] (1) Dissolve the cryoprecipitate with 0.02M tromethamine (Tris) buffer, precipitate with 30% polyethylene glycol, centrifuge, and obtain the supernatant;
[0062] (2) After the supernatants were combined and clarified, Tween-80 and tributyl phosphate were added to make the final concentrations 1% and 0.3%, respectively, and treated at 25°C±1°C for 6 hours to complete the first virus inactivation (that is, SD virus inactivated);
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com