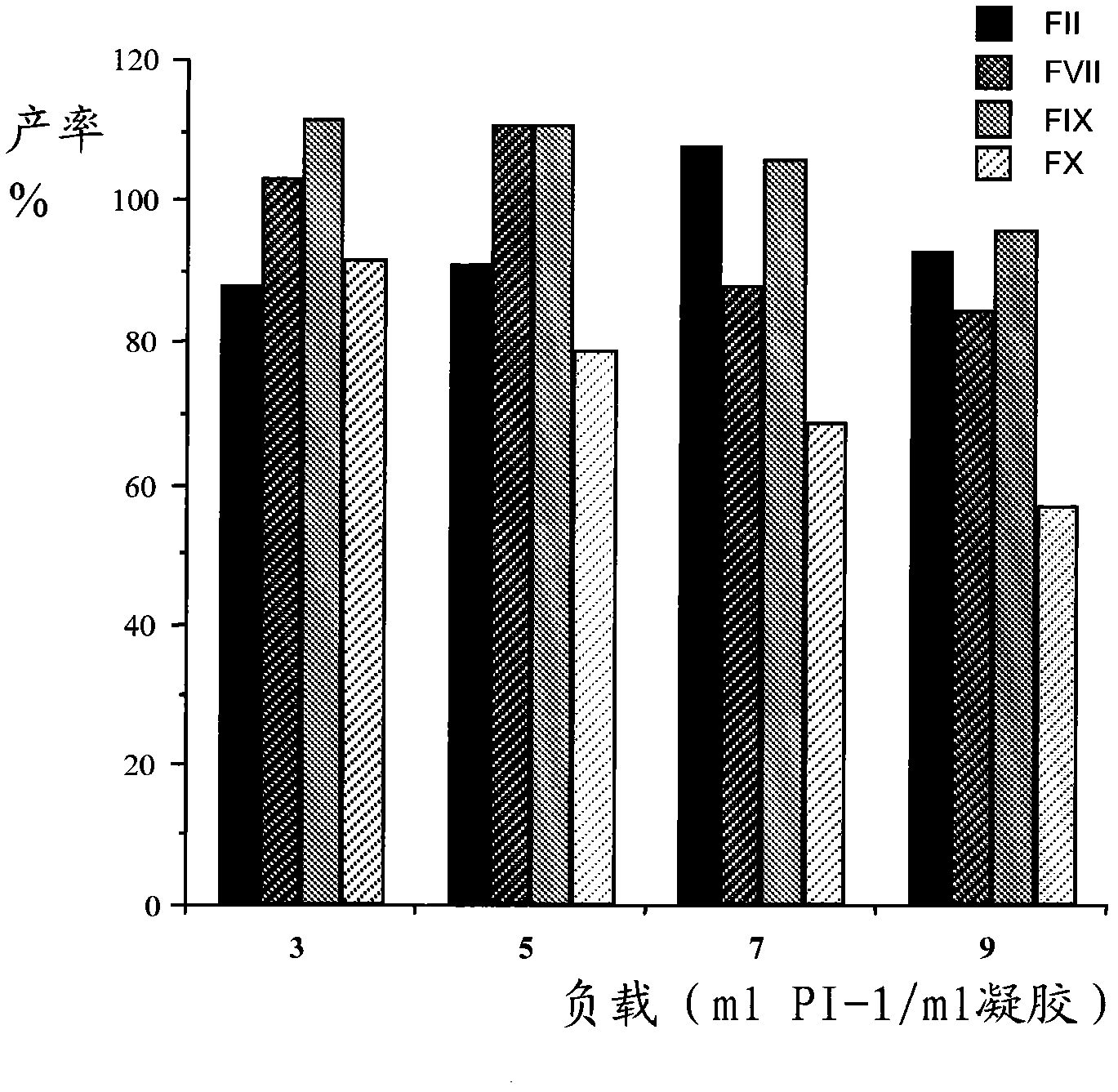
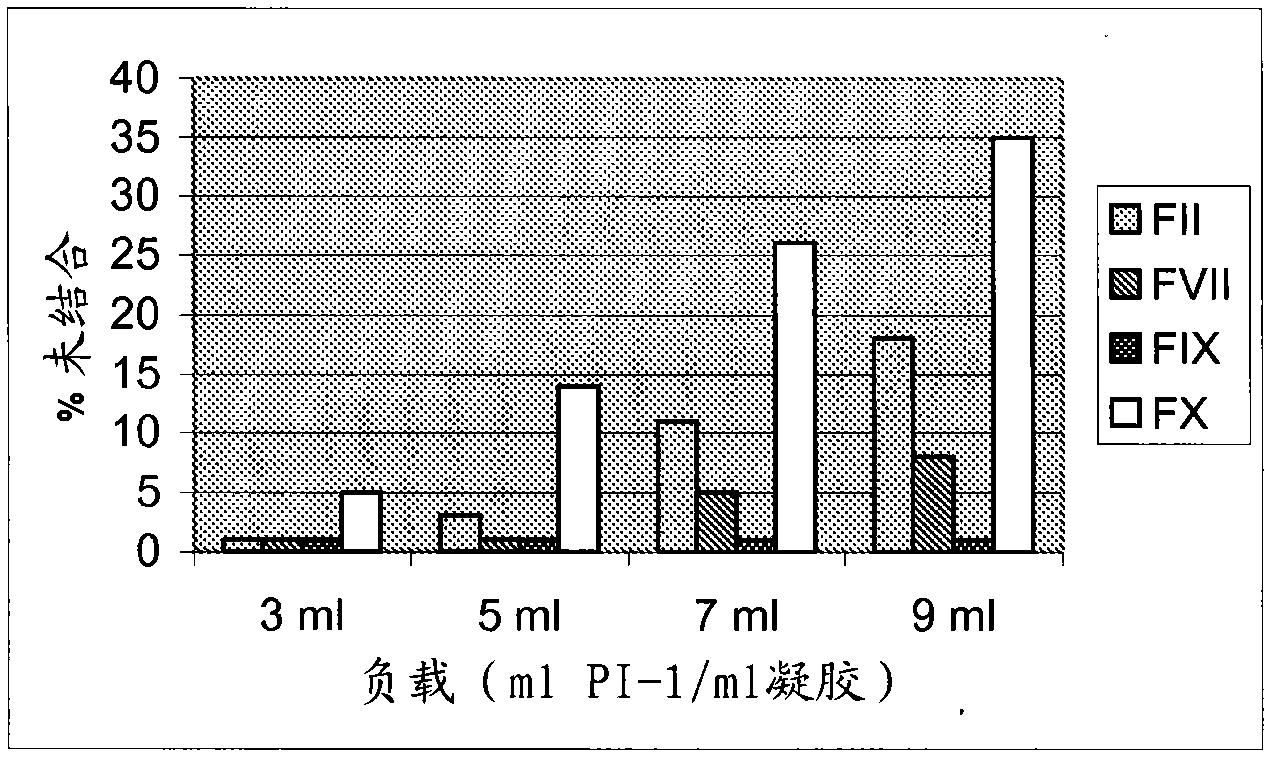
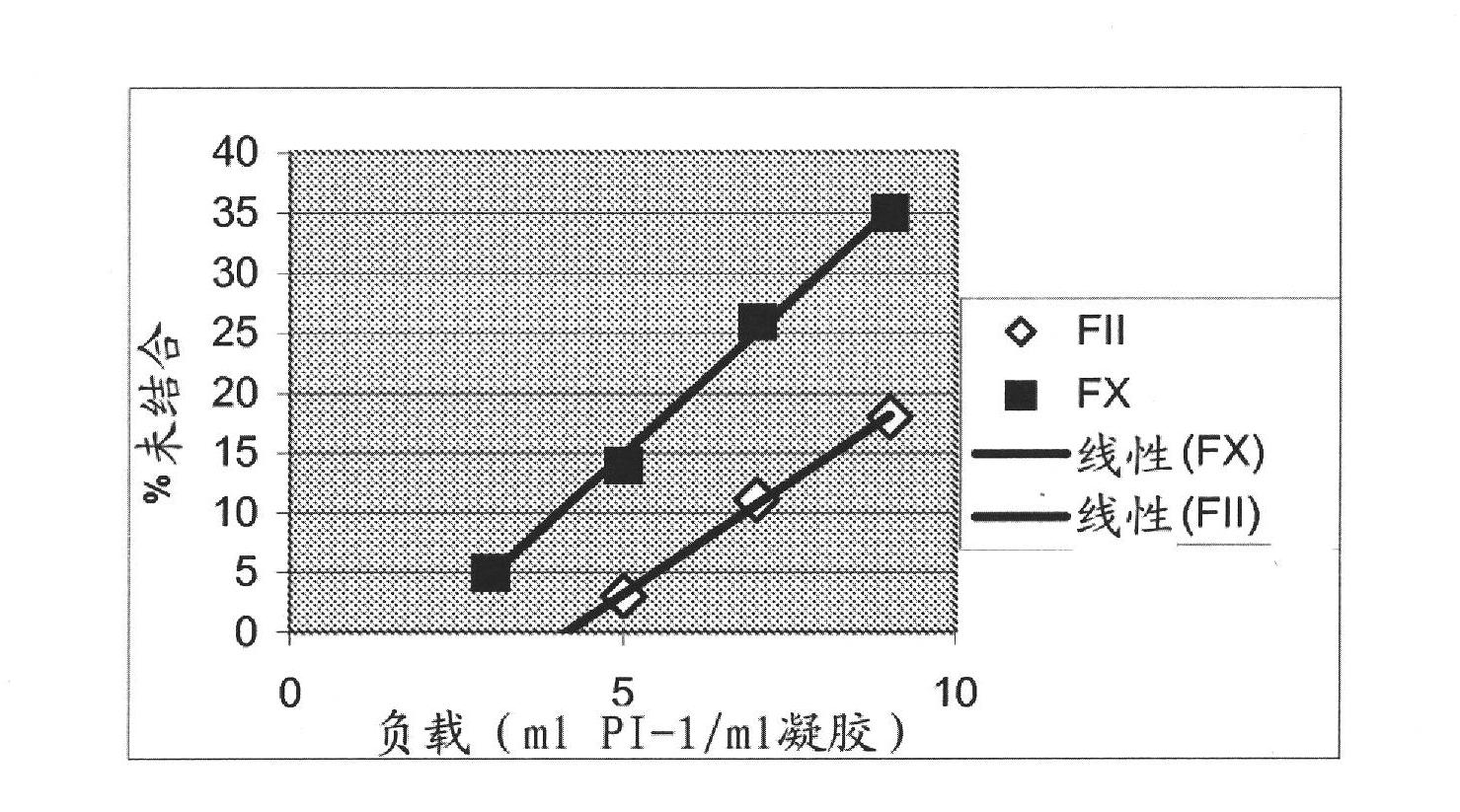
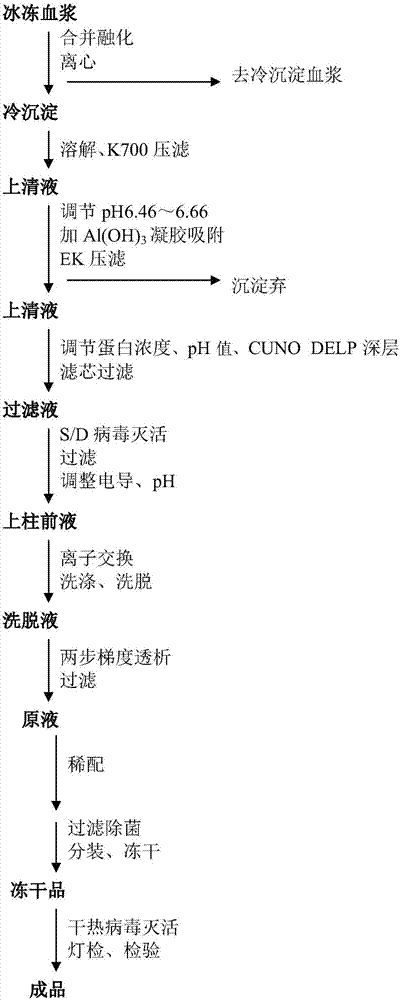
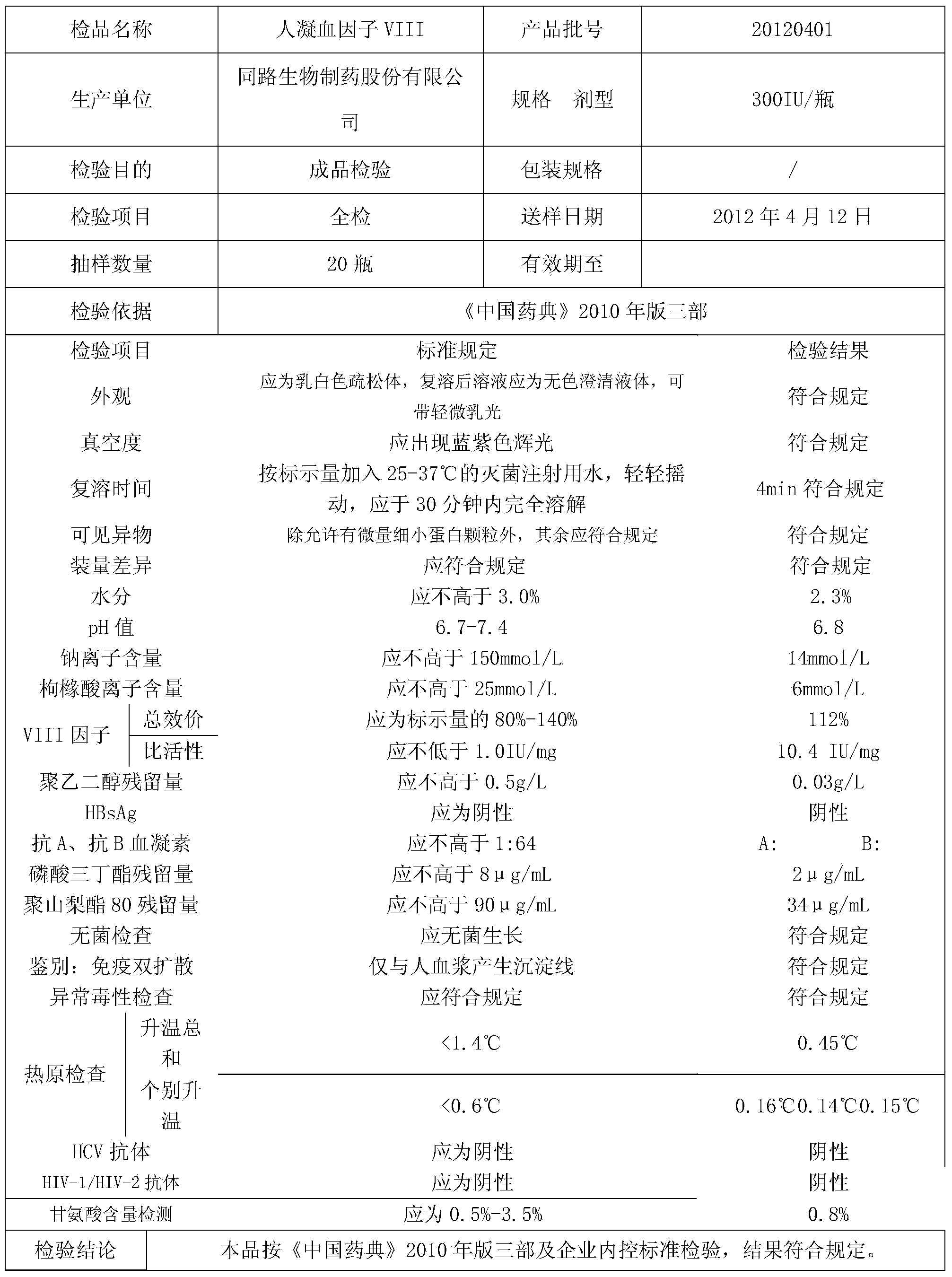
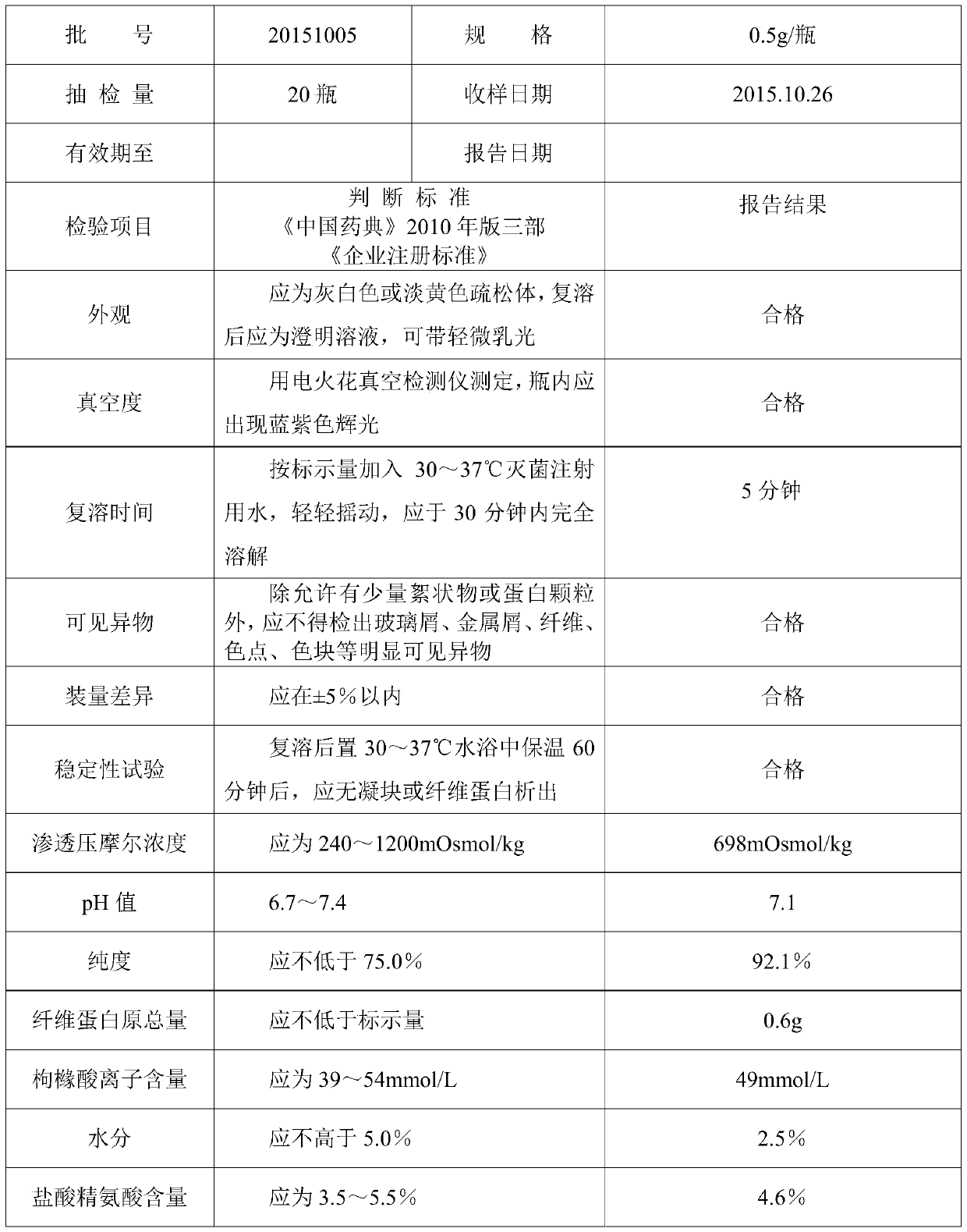
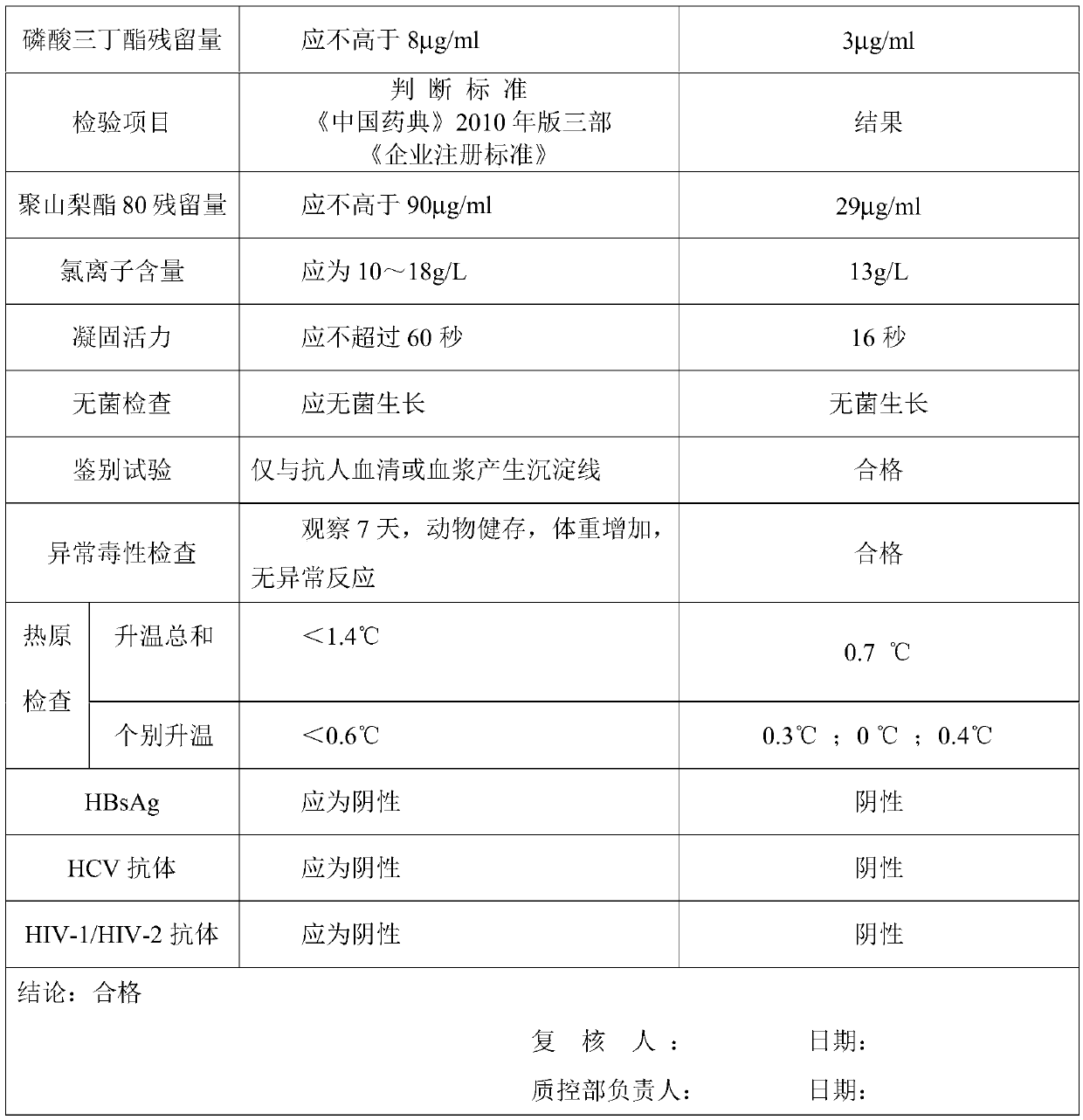
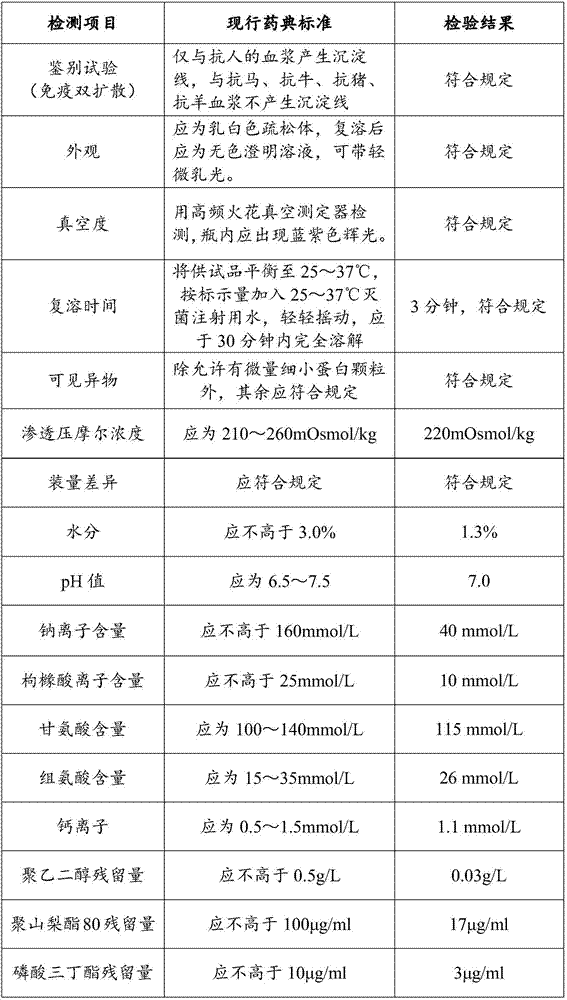
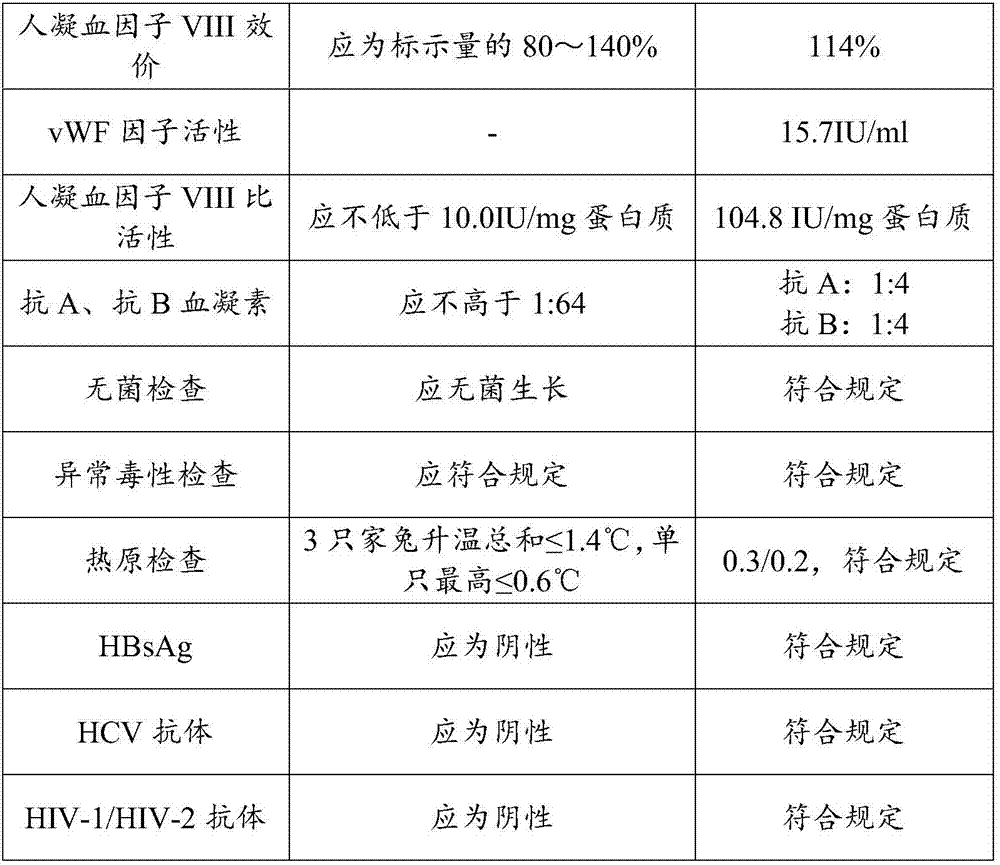
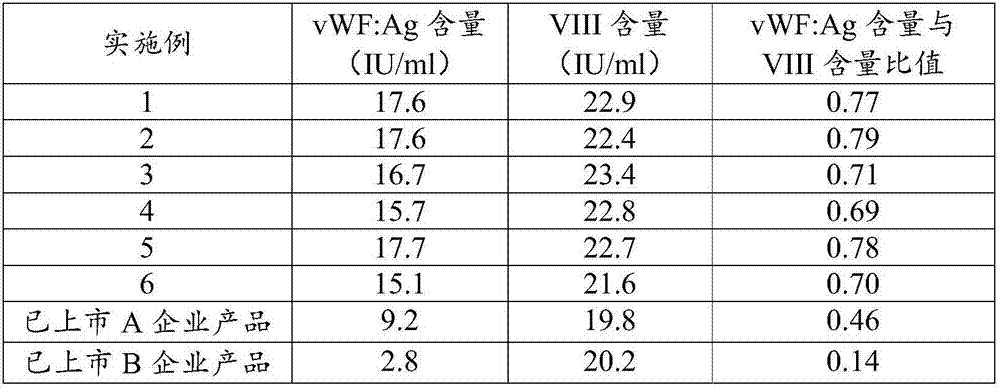
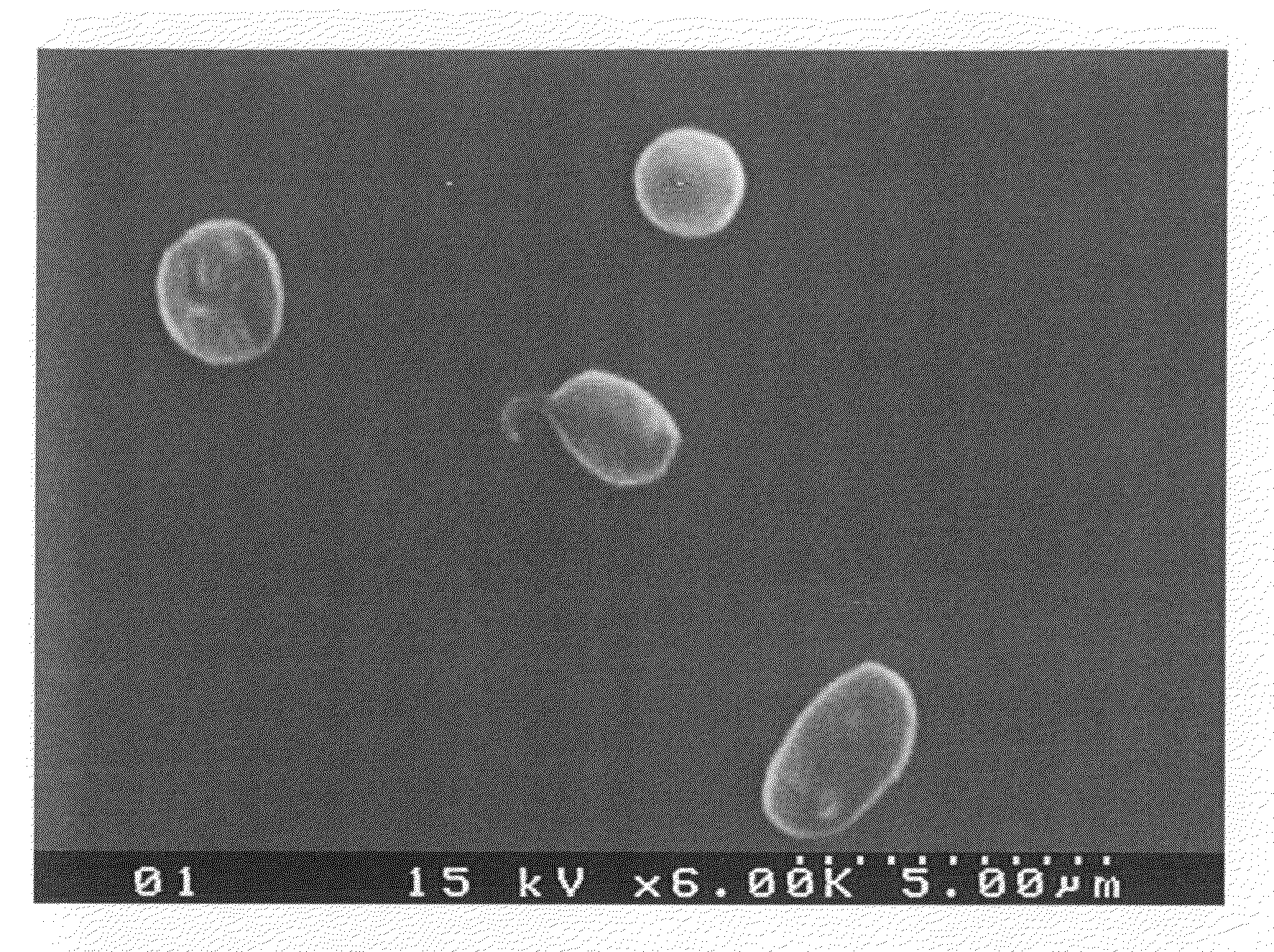Patents
Literature
40 results about "Cryoprecipitated AHF" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
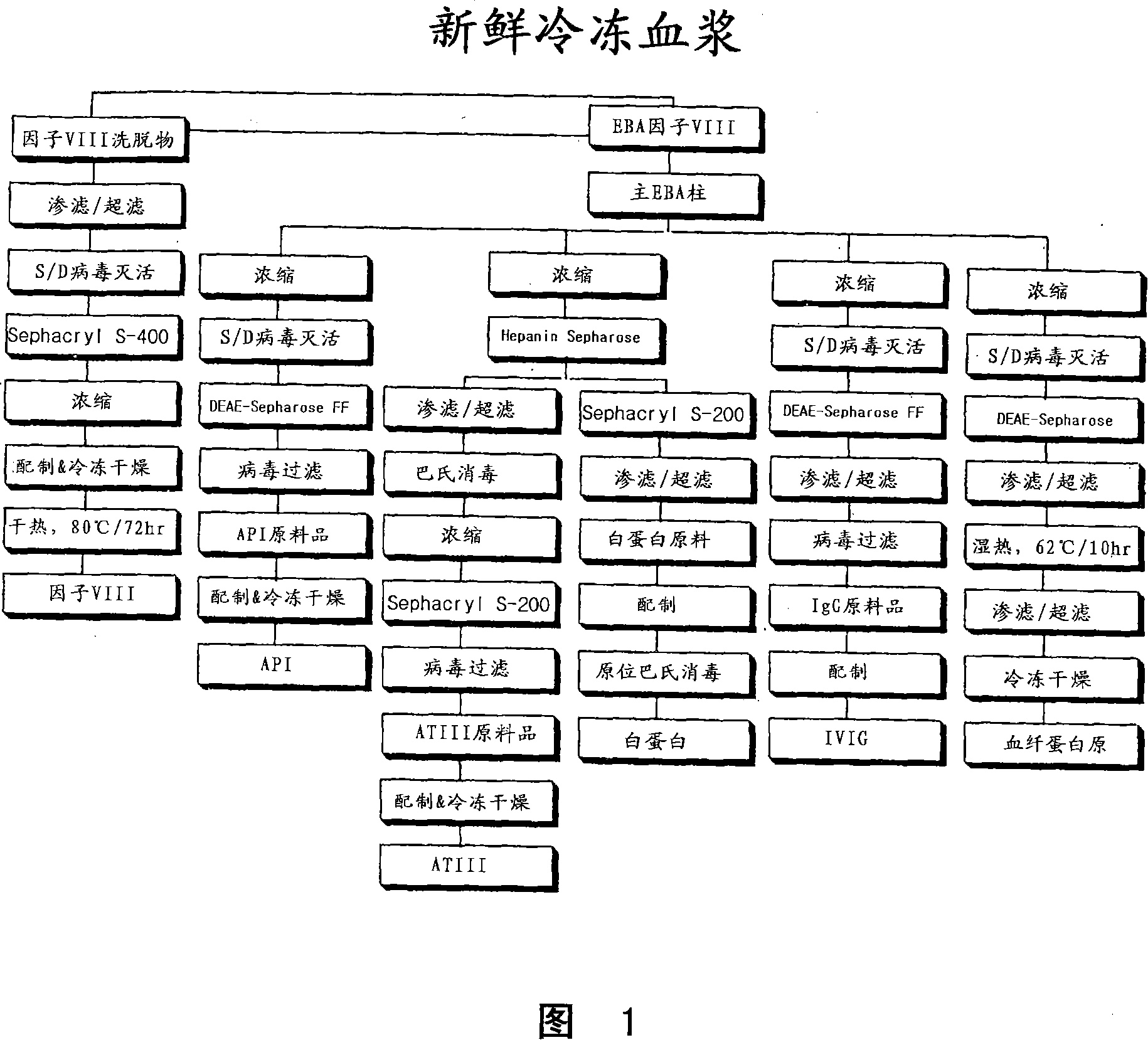
It is often transfused as a four-to six-unit pool instead of as a single product. One of the most important constituents is factor VIII (also called antihaemophilic factor or AHF), which is why cryoprecipitate is sometimes called, or refined into, cryoprecipitated antihaemophilic factor or cryoprecipitated AHF.
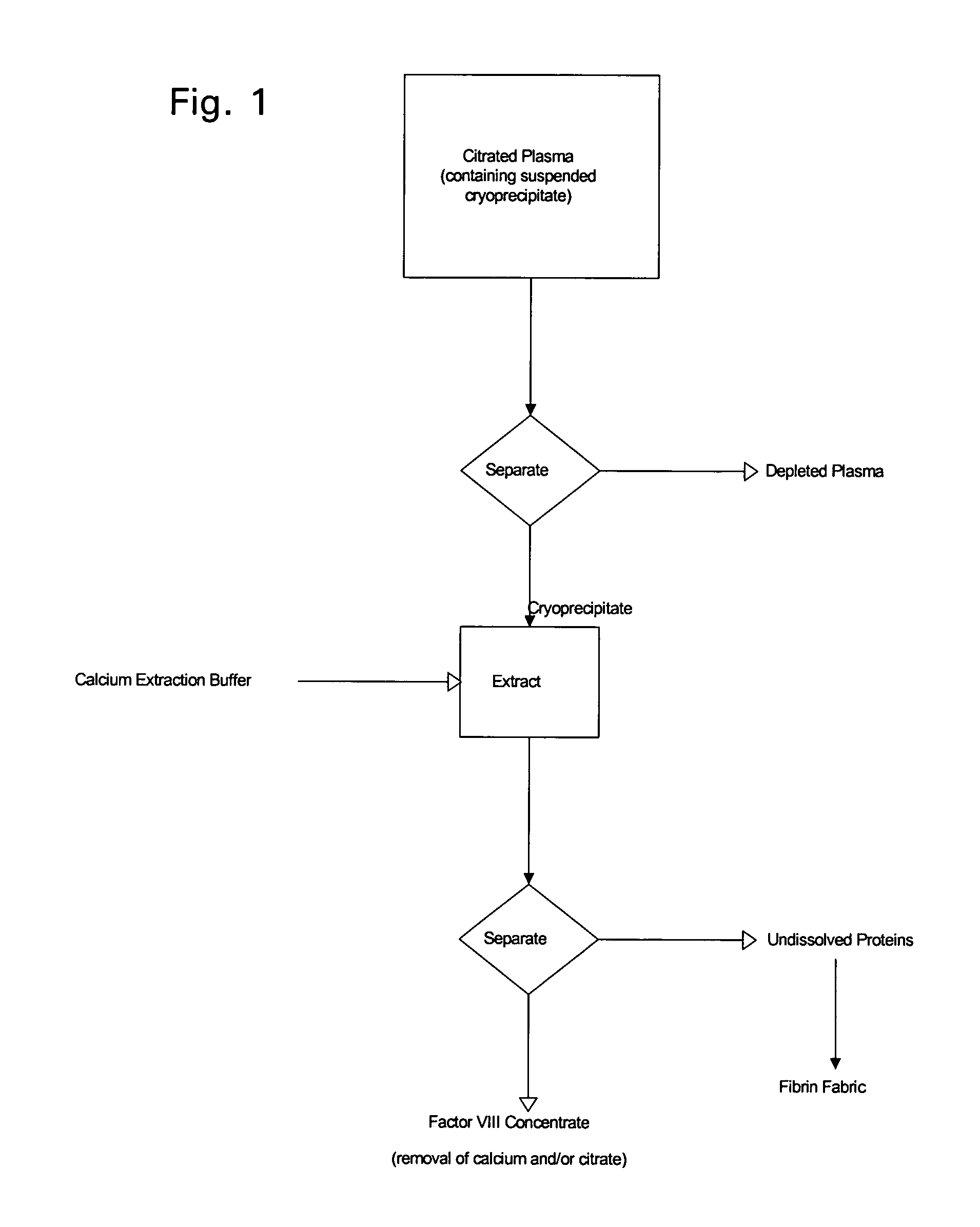
Enhanced production of blood clotting factors and fibrin fabric
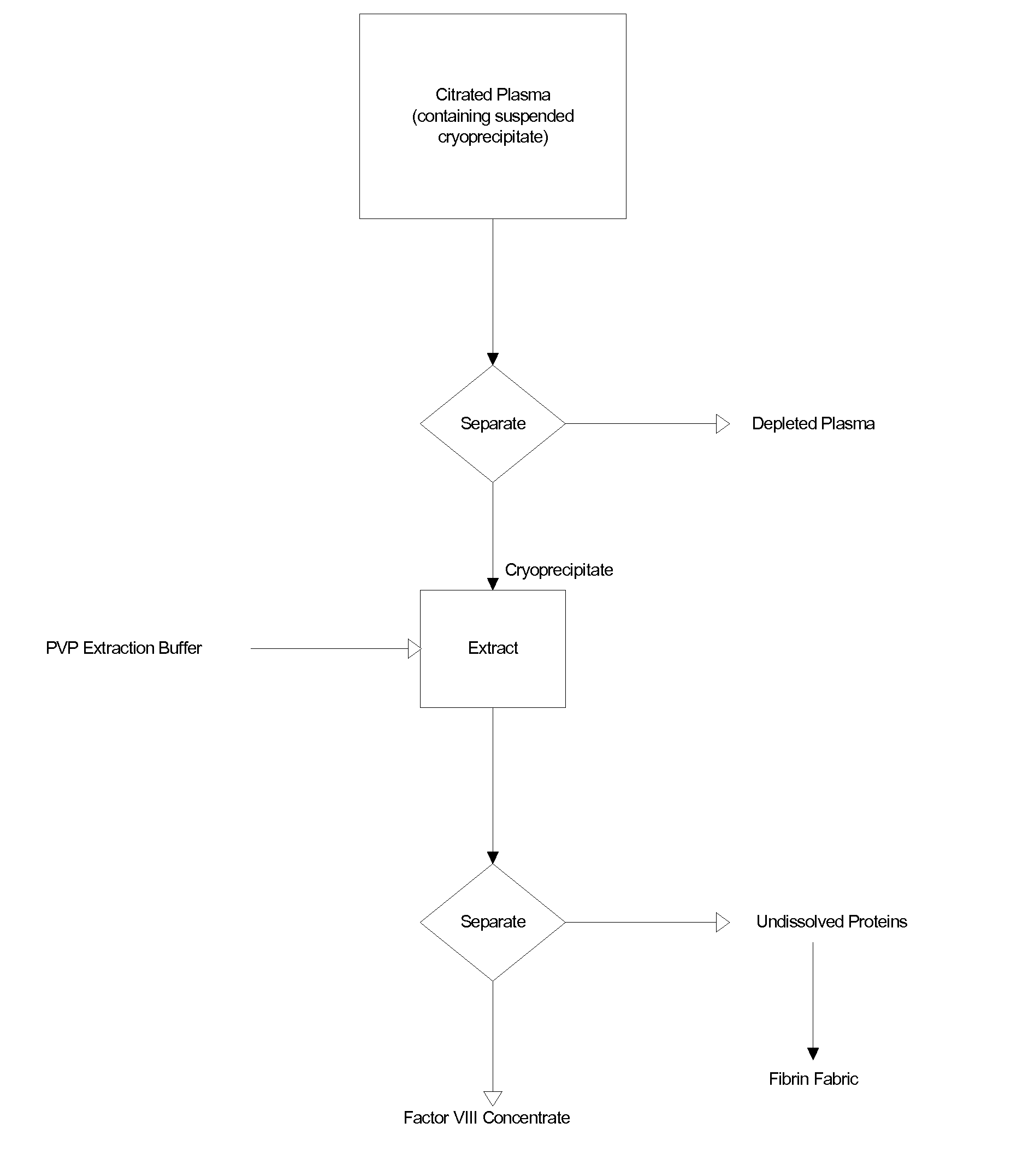
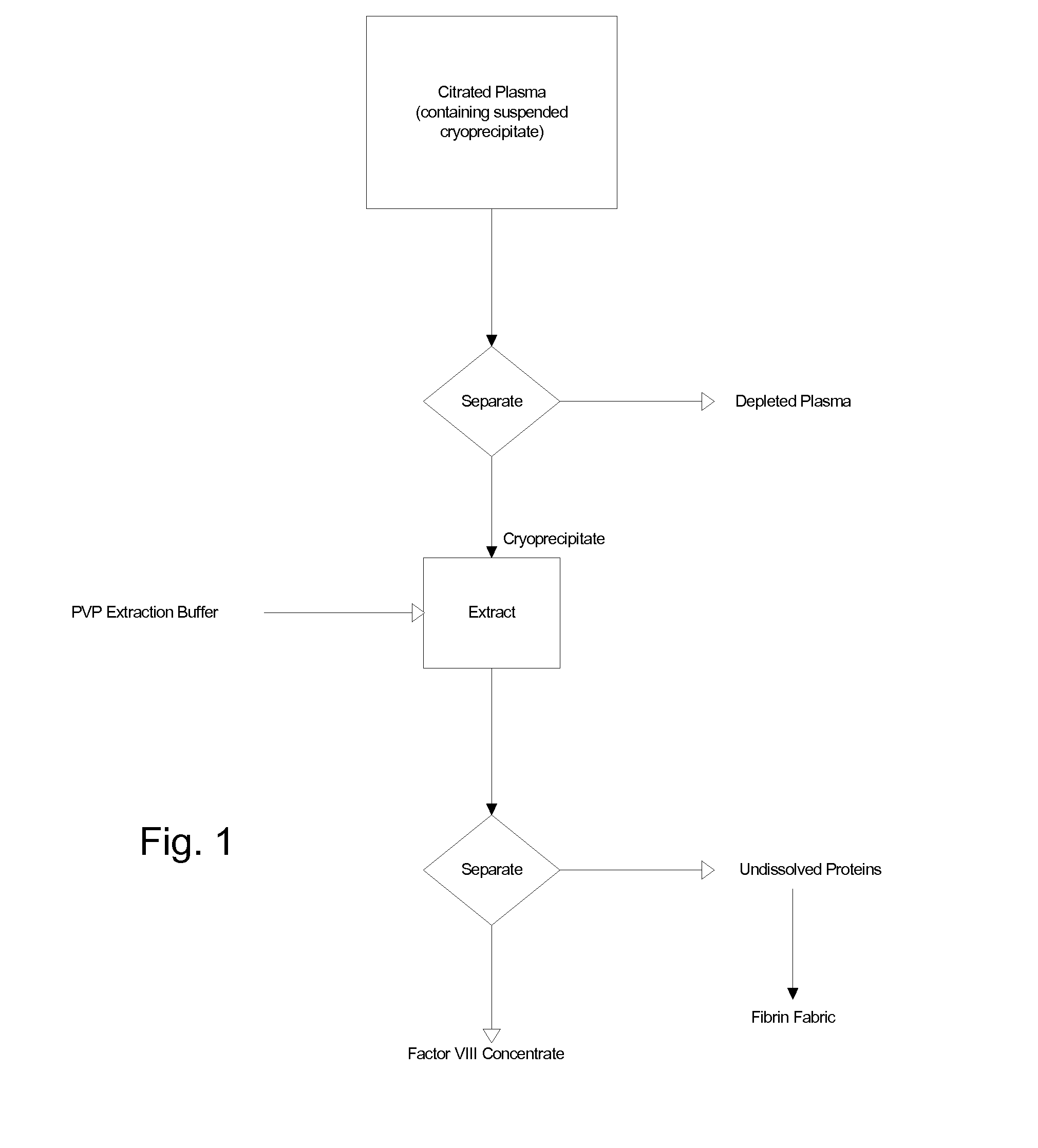
The blood collection, processing and transfer by separation of discrete components containing additional citrate (at least about trisodium citrate 9% w / v) in one or other of collection or processing bag provides for enhanced yield and purity of cryoprecipitate. Inhibiting the activation or denaturation of blood components including blood cells and plasma proteins and with the removal of the activated and denatured components thereby improving safety and efficacy of end products. The inventive process is particularly suited to an improved extraction process to yield concentrated clotting factors from single donors or limited pools without use of chromatography. Following extraction the remaining cryoprecipitate can advantageously be formed into a fibrin fabric used in surgeries and in the treatment of wounds.
Owner:SHANBROM TECH
Method for stabilizing a cryoprecipitate of plasmatic proteins for being subjected to a viral inactivation thermal treatment
ActiveUS20060247426A1Shorten the timeImprove protectionPowder deliveryPeptide/protein ingredientsCryoprecipitateMedicine
The invention relates to a method for obtaining cryoprecipitatable proteins, comprising a viral inactivation step by thermally treating a lyophilisate of these proteins, comprising, before rendering the proteins in the form of a lyophilisate, an initial addition step, to these proteins, of a stabilizing and solubilizing formulation containing a mixture consisting of arginine, at least one hydrophobic amino acid and of tribasic sodium citrate. The invention also relates to a concentrate consisting of at least one cryoprecipitable protein containing the stabilizing and solubilizing formulation introduced according to the method and being suited for therapeutic use.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
Compositions Suitable for Treatment of Spinal Disease, Disorder or Condition
InactiveUS20100310524A1Raise the ratioRestore disc heightBiocideNervous disorderCryoprecipitateMedicine
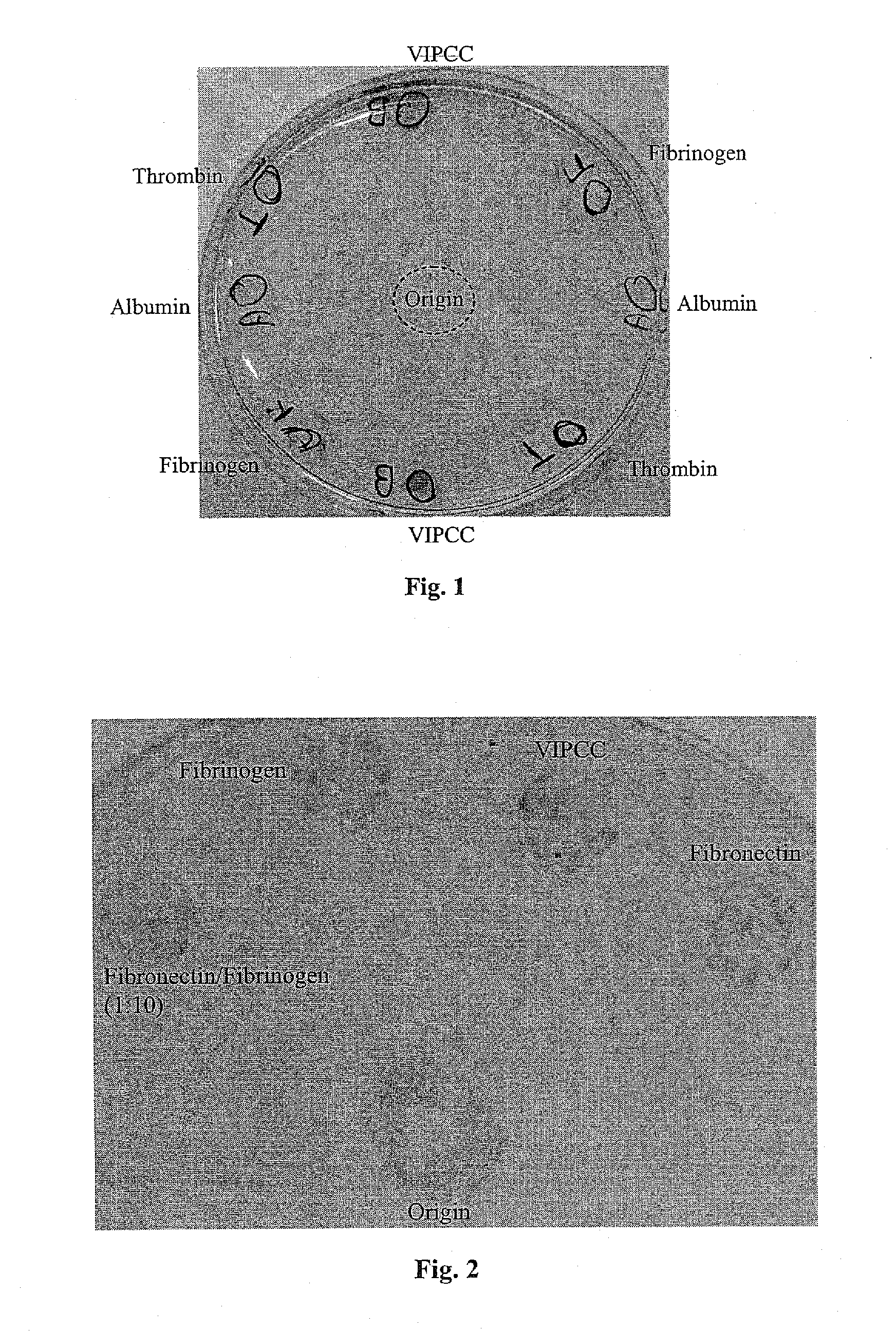
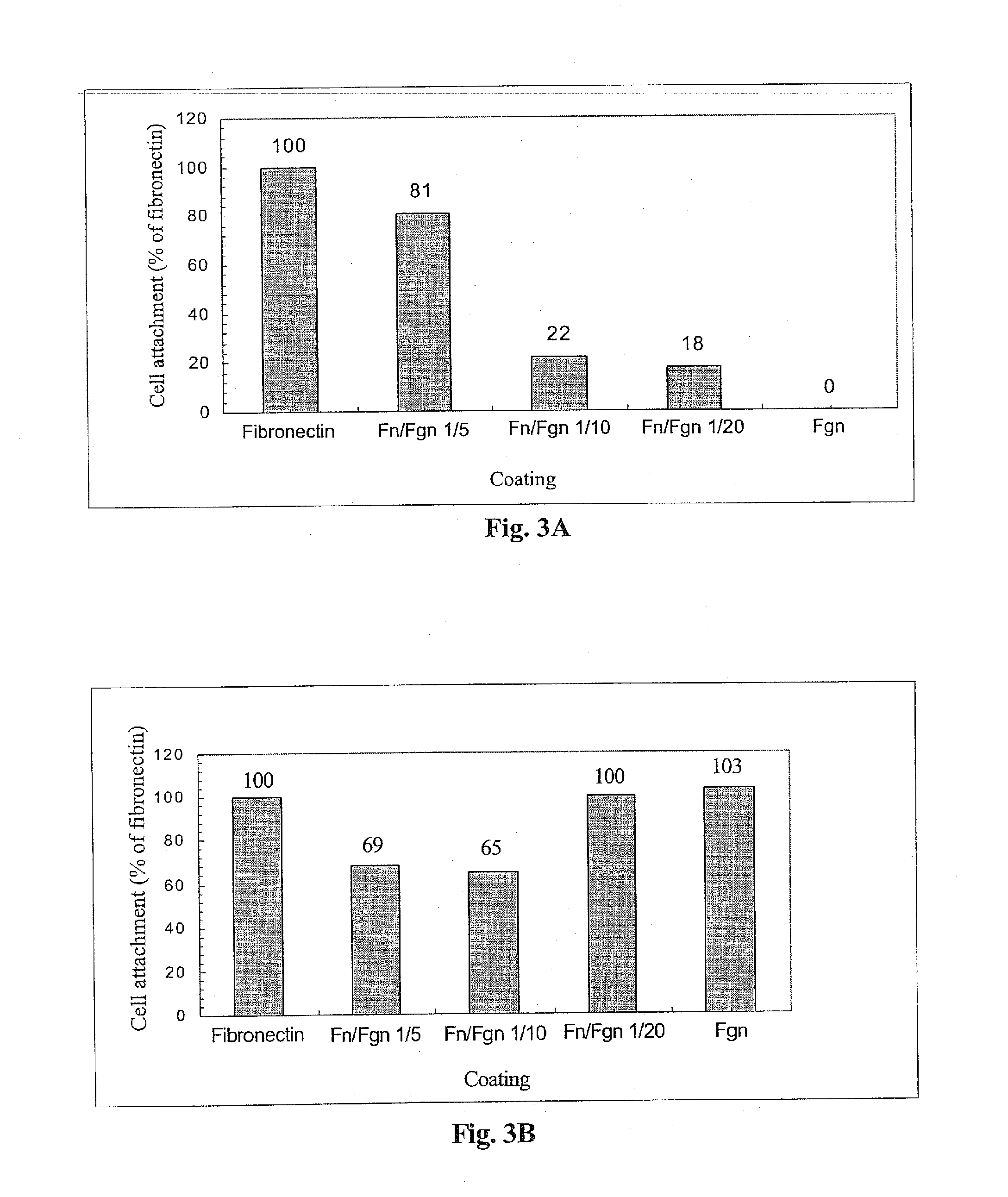
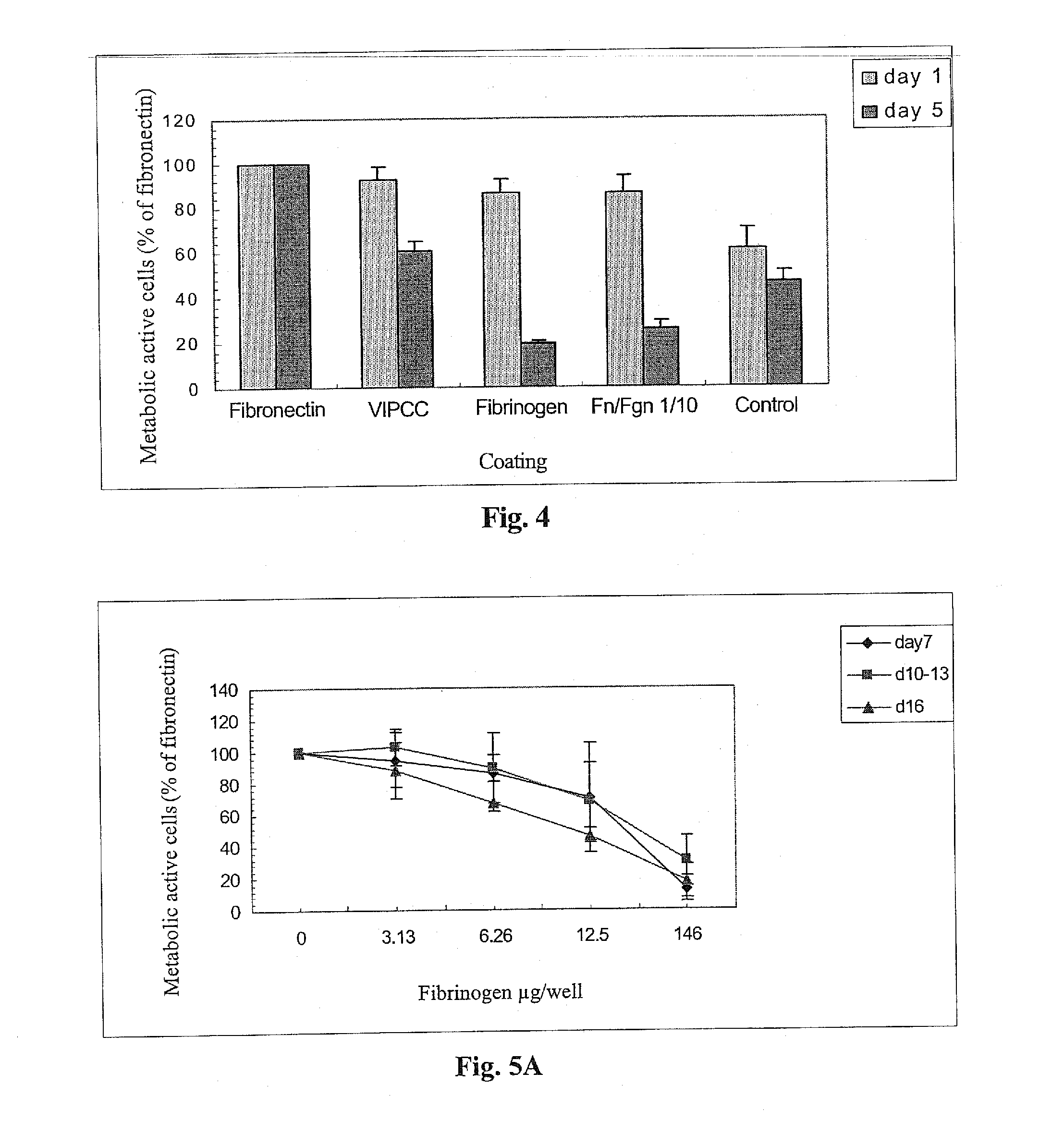
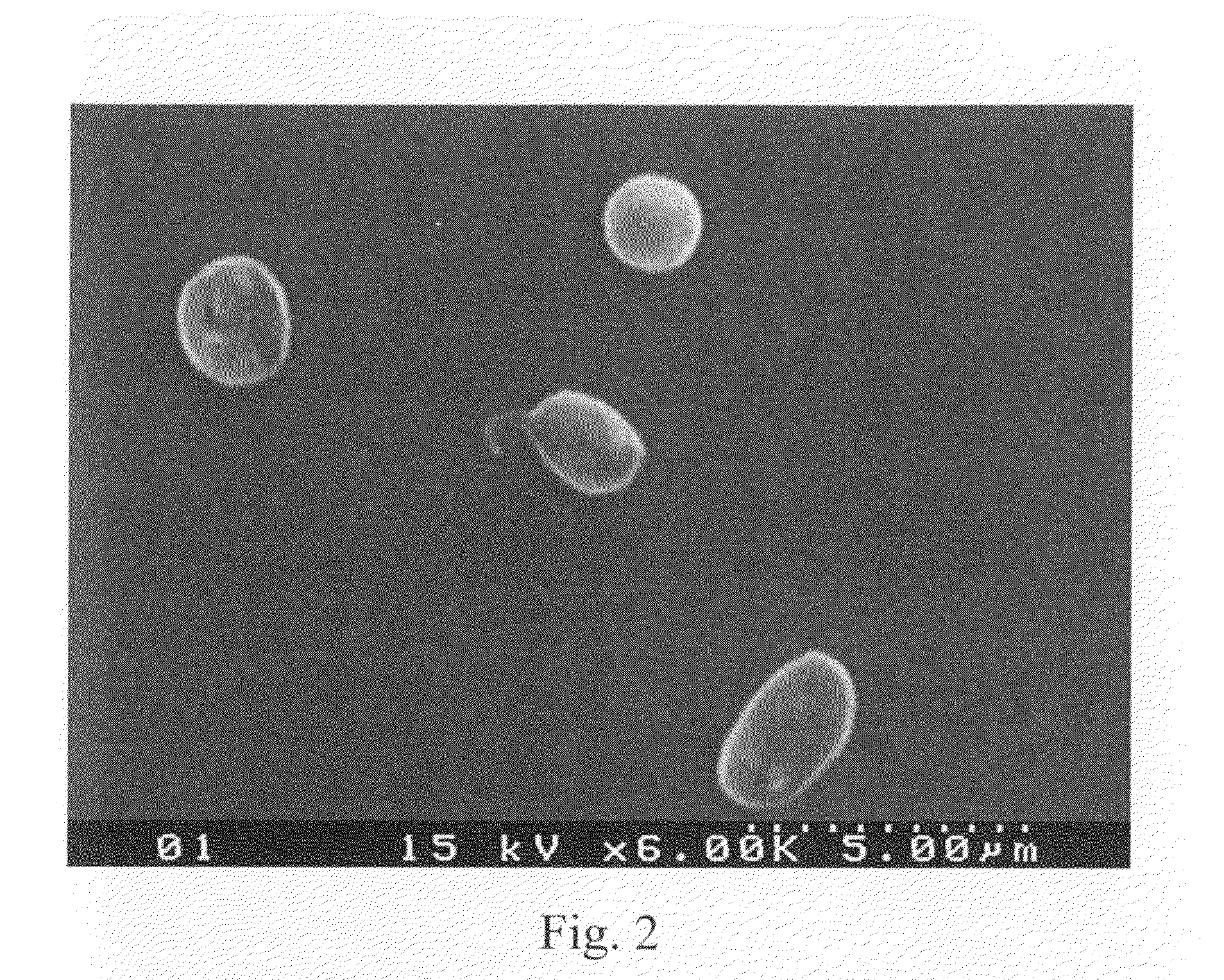
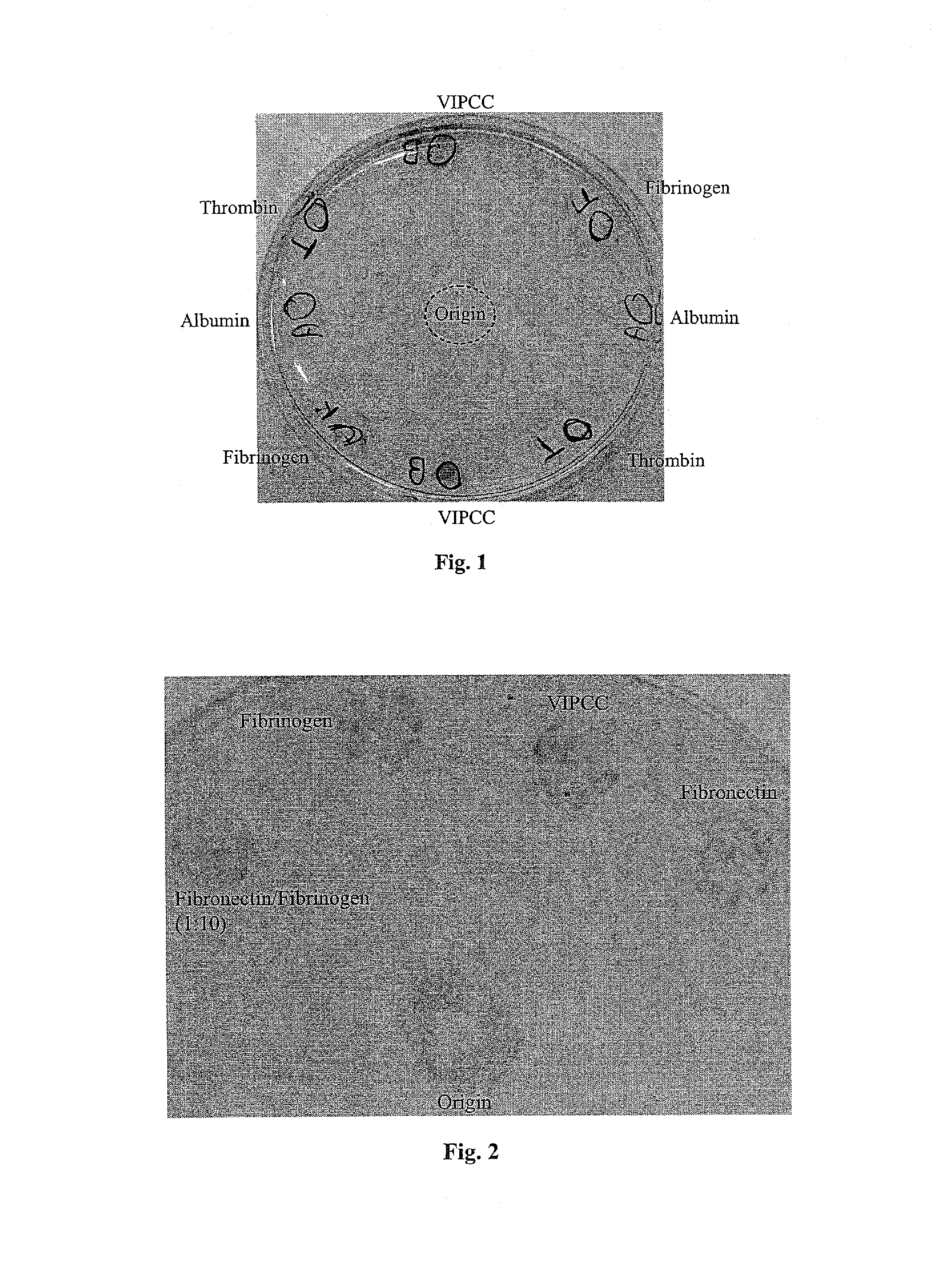
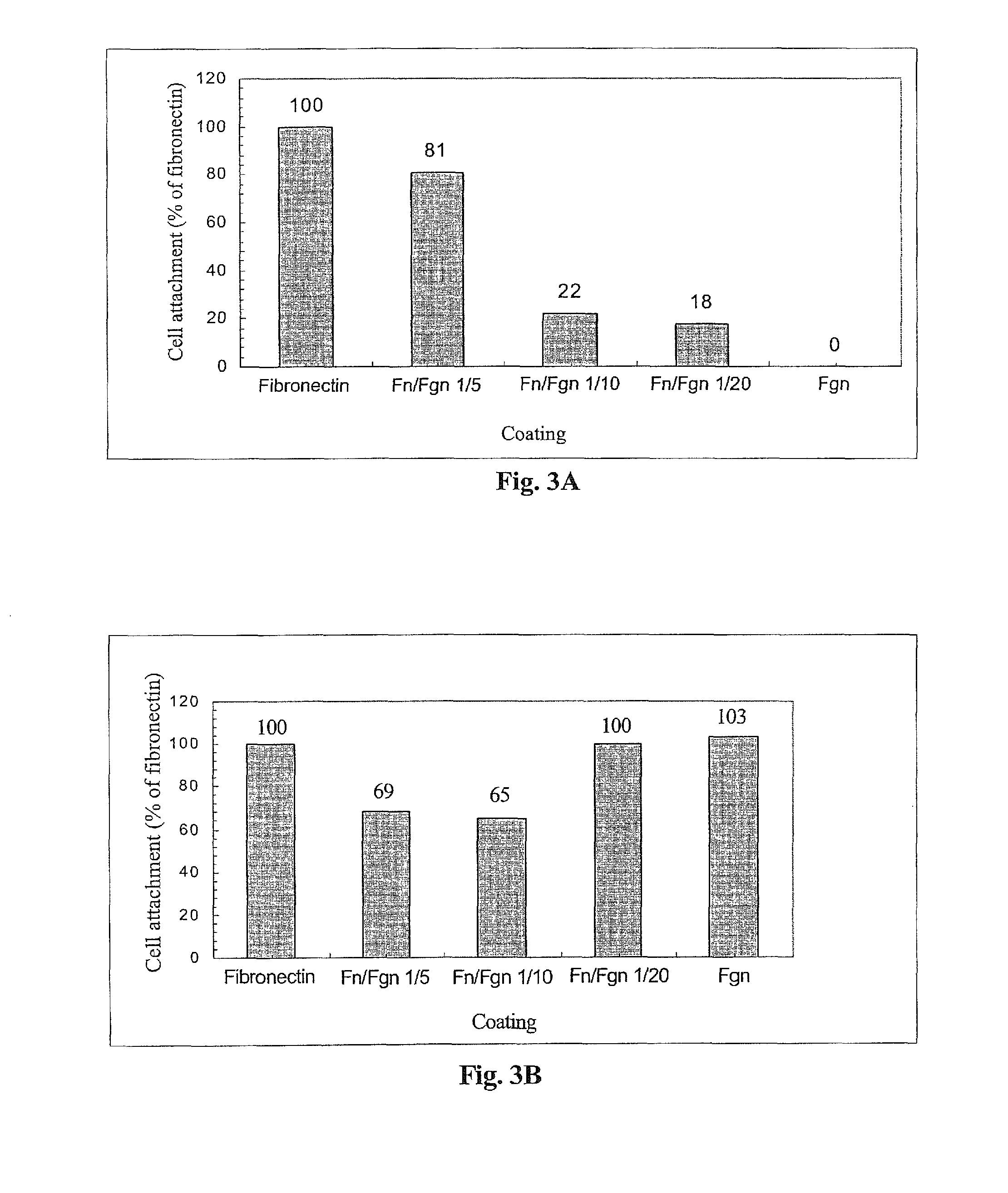
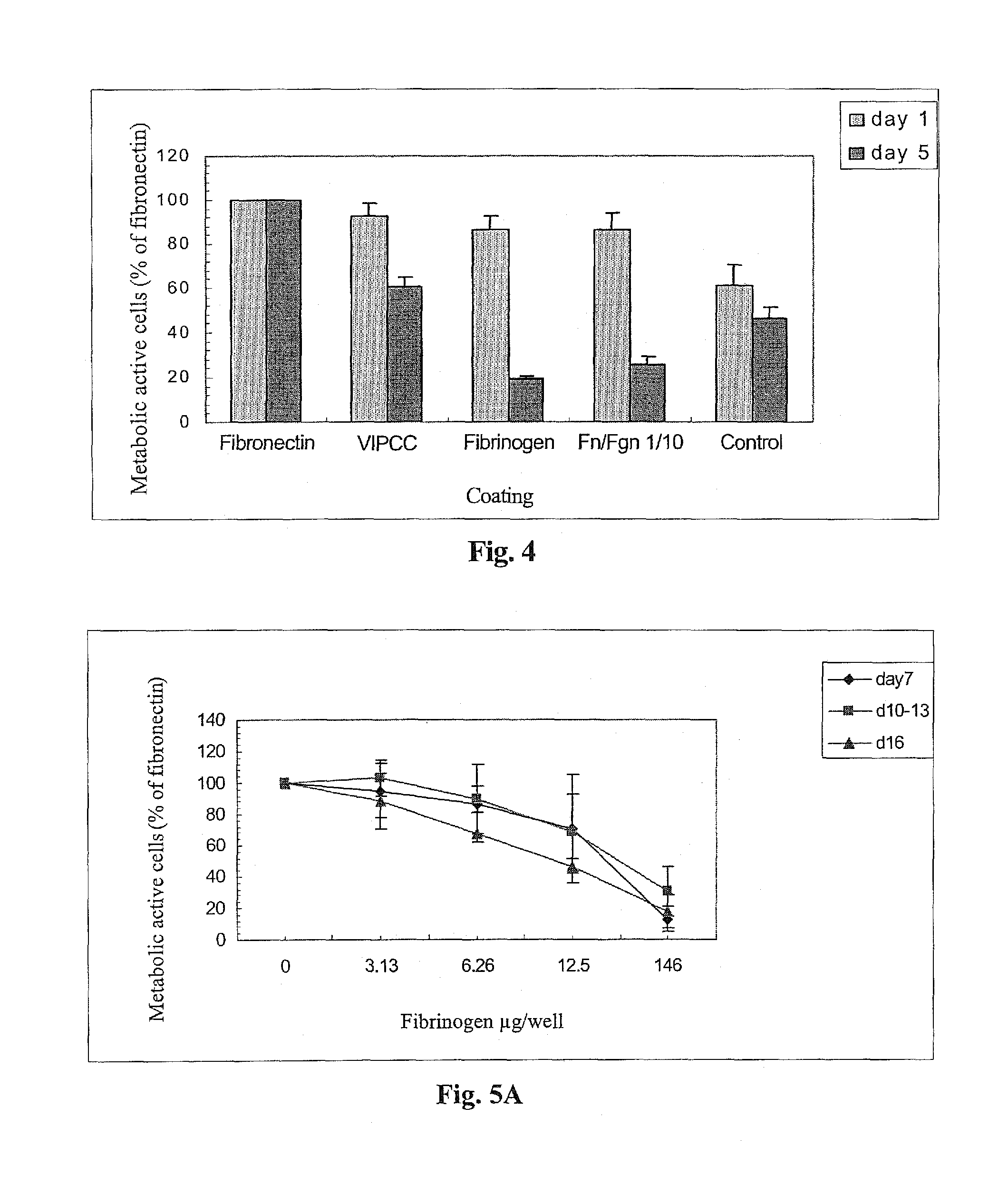
The invention relates to the use of viral inactivated-plasma cryoprecipitate concentrate (VIPCC) comprising a suitable fibronectin / fibrinogen ratio for treating a spine disease, disorder or condition such as intervertebral disc degeneration.
Owner:OMRIX BIOPHARM
Prothrombic complex composition
InactiveCN102459583AMicrobiological testing/measurementInactivation/attenuationCryoprecipitateIon-exchange resin
The present invention relates to a method for preparing a composition or a concentrate of a prothrombic complex that includes the II, VII, IX and X coagulation factors, wherein said method includes the steps of providing a supernatant of a plasma cryoprecipitate, applying said supernatant on an anion-exchange resin in order to produce an eluate containing said complex and proteins having a high molecular weight, and applying said eluate on a hydroxyapatite column in order to produce a second eluate containing said complex. The invention also relates to a composition that can be produced by said method.
Owner:法国分馏学和生物学实验室
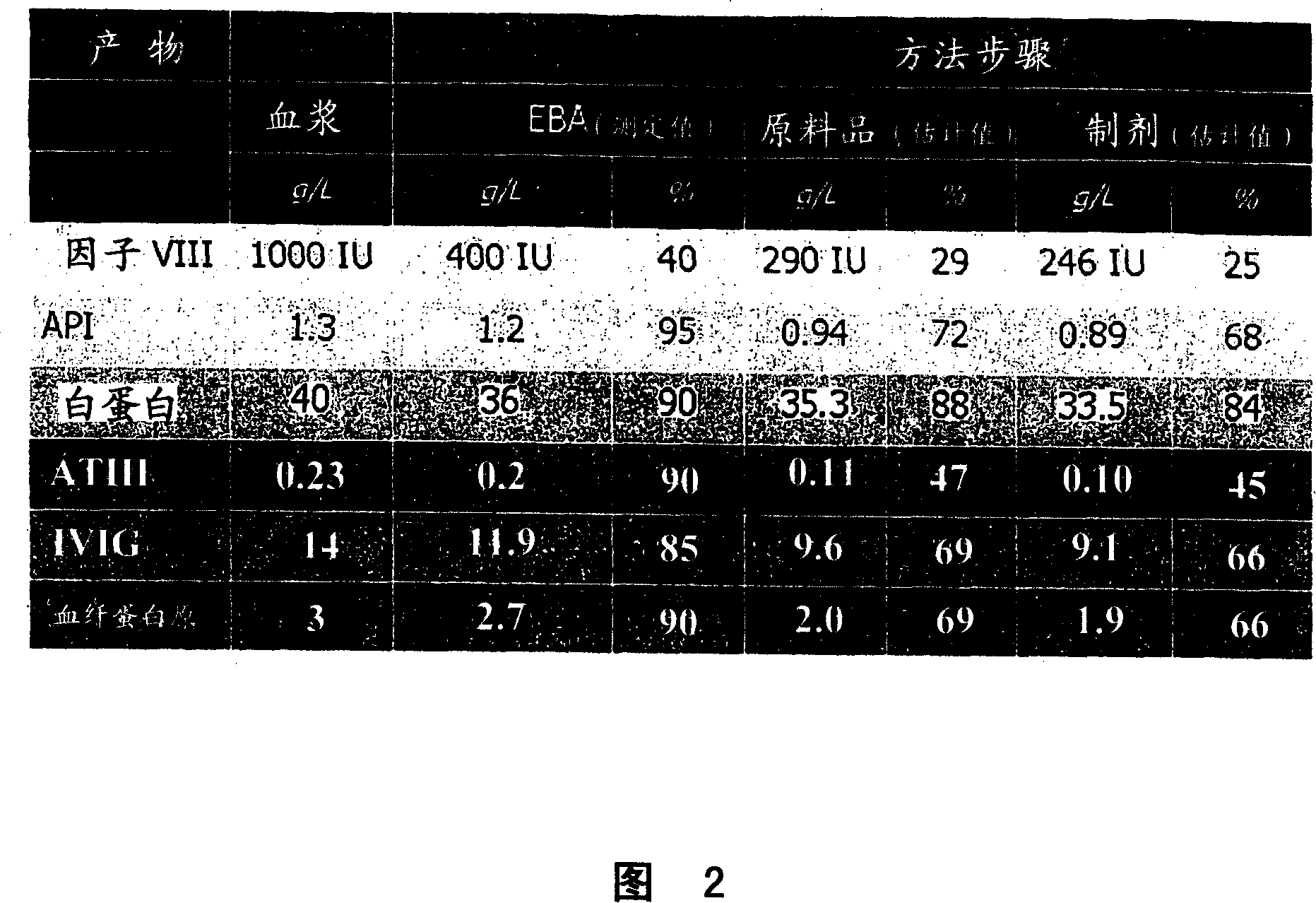
Human coagulation factor VIII preparation method
ActiveCN107226859AImprove securityHigh potencyFactor VIIPeptide preparation methodsFreeze-dryingDissolution
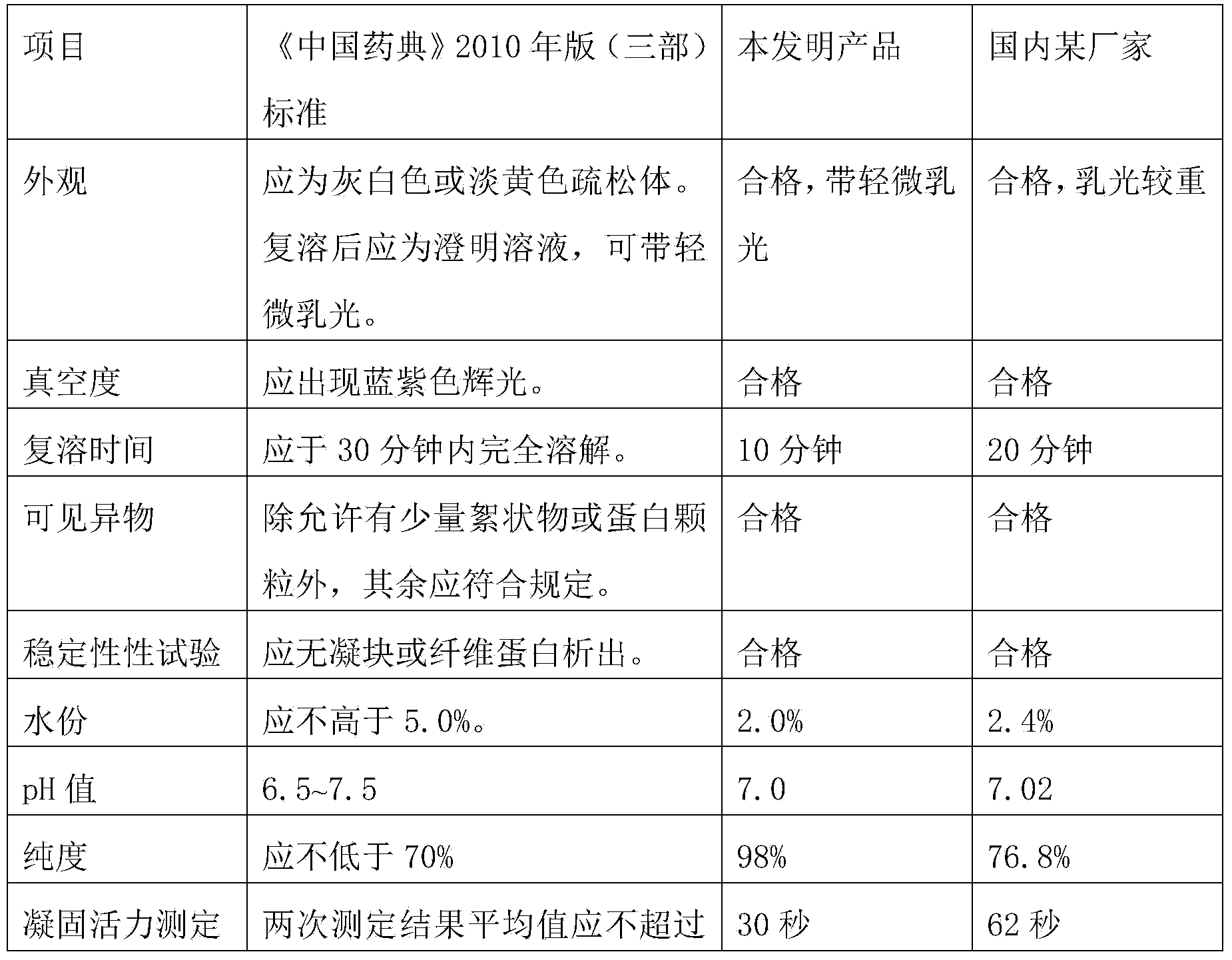
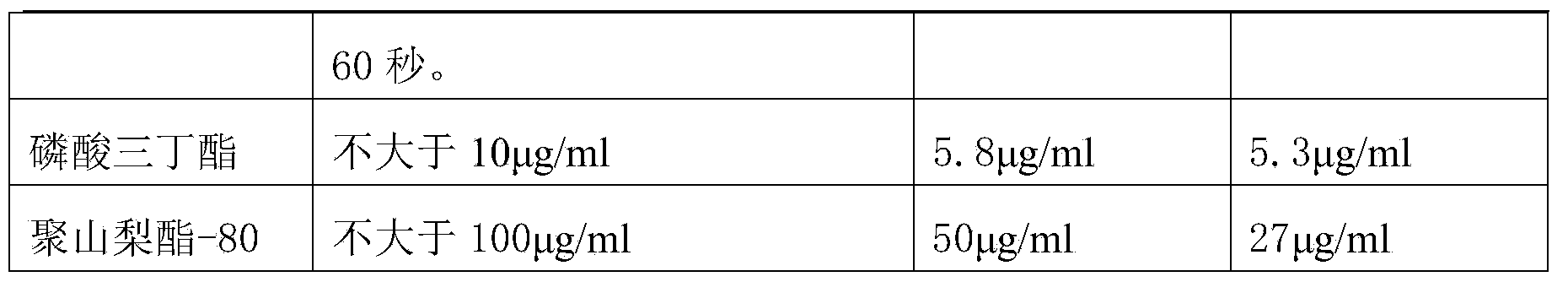
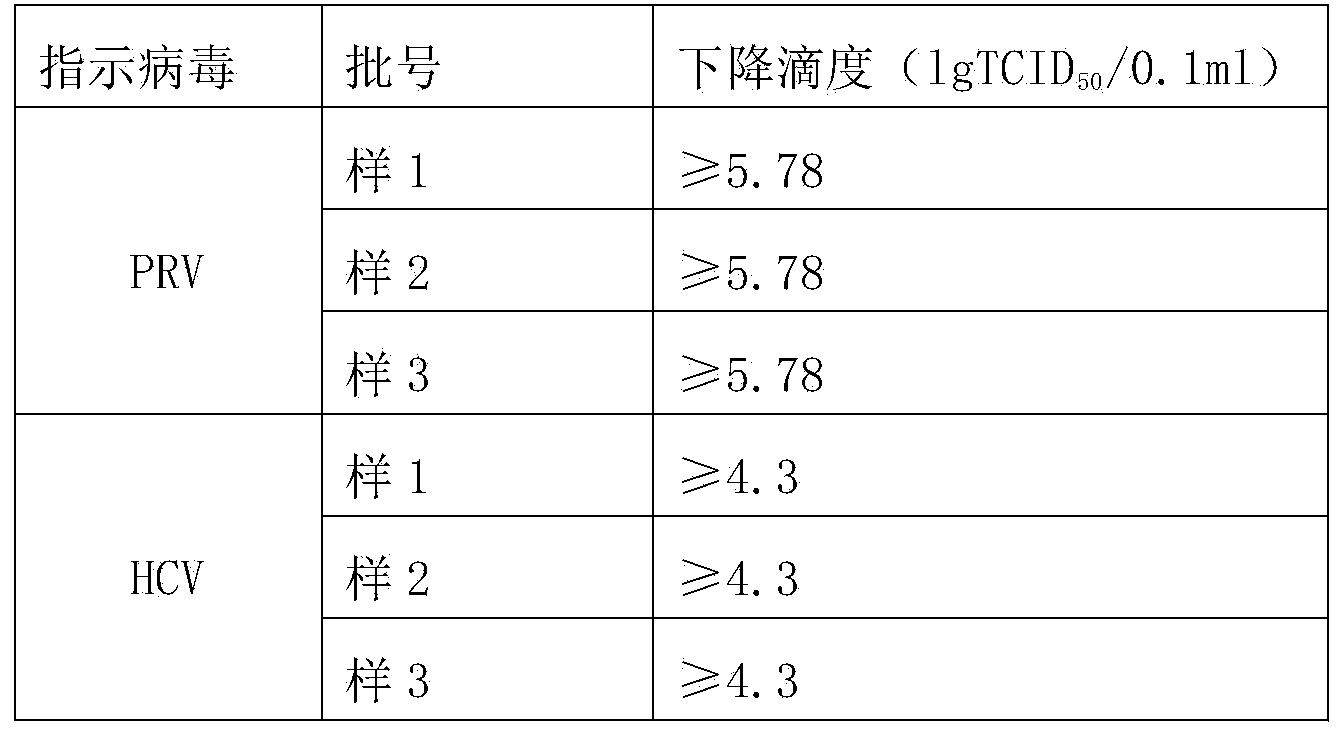
The invention discloses a human coagulation factor VIII preparation method. In the preparation process of a human coagulation factor VIII, a two-step filter press technique is adopted during treatment of human plasma initial materials, that is, a K700 filter plate is adopted for filter pressing after cryoprecipitate dissolution, the filter press liquor is collected, the pH value is adjusted, a 2% aluminium hydroxide gel is added for adsorption, and then an EK filter plate is adopted for filter pressing. The two-step filter press technique is adopted to improve the separation effect, meanwhile, the aluminium hydroxide gel adsorption method is used together to remove a coagulation factor on which vitamin K depends, the aluminium hydroxide gel does not adsorb the human coagulation factor VIII, and the product yield is high. In the preparation process, the two-step filter press technique replaces a traditional two-step high-speed centrifuging method, a CUNO DELP deep filter element is adopted for filtration, a two-step gradient dialysis method is adopted, a re-dissolution and re-freezing process is added to a freeze-drying process, and meanwhile, various virus removal / inactivation technologies are adopted in the production process, so that the product yield can be improved, the risk of virus spreading can be reduced, and the clinical medication security is improved.
Owner:华润博雅生物制药集团股份有限公司
Technological process for improving stable FVII yield of human prothrombin complexes
ActiveCN101439047AImprove bindingHigh yieldPowder deliveryPeptide/protein ingredientsFiltrationUltrafiltration
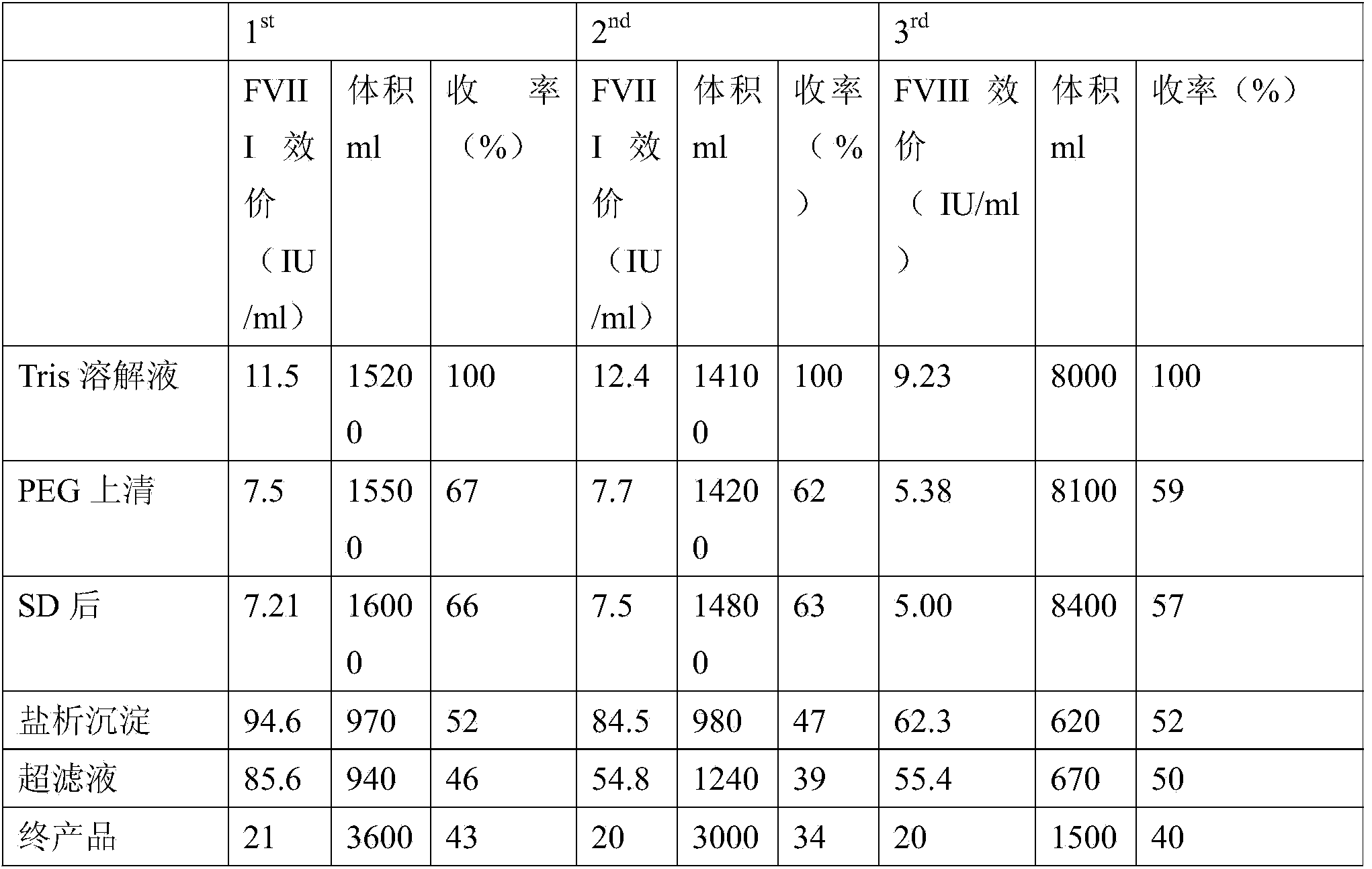
The invention relates to the field of bio-pharmaceutical technology, in particular to a technical method for improving stable F VII yield rate of human thrombogen complex. The following procedures are included: freshly freezing blood plasma of a healthy person at a low temperature; removing cryoprecipitate; carrying out clarification filtration; adjusting ionic strength; carrying out gel absorption; carrying out wash and elution; carrying out ultrafiltration; killing S / D virus; carrying out gel absorption; carrying out wash and elution; carrying out ultrafiltration; carrying out aseptic filtration and subpackage; carrying out freeze-drying; and carrying out hot-air sterilization.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD +1
Medium comprising cryoprecipitate and method for preserving platelets, red blood cells and other cells without a nucleus
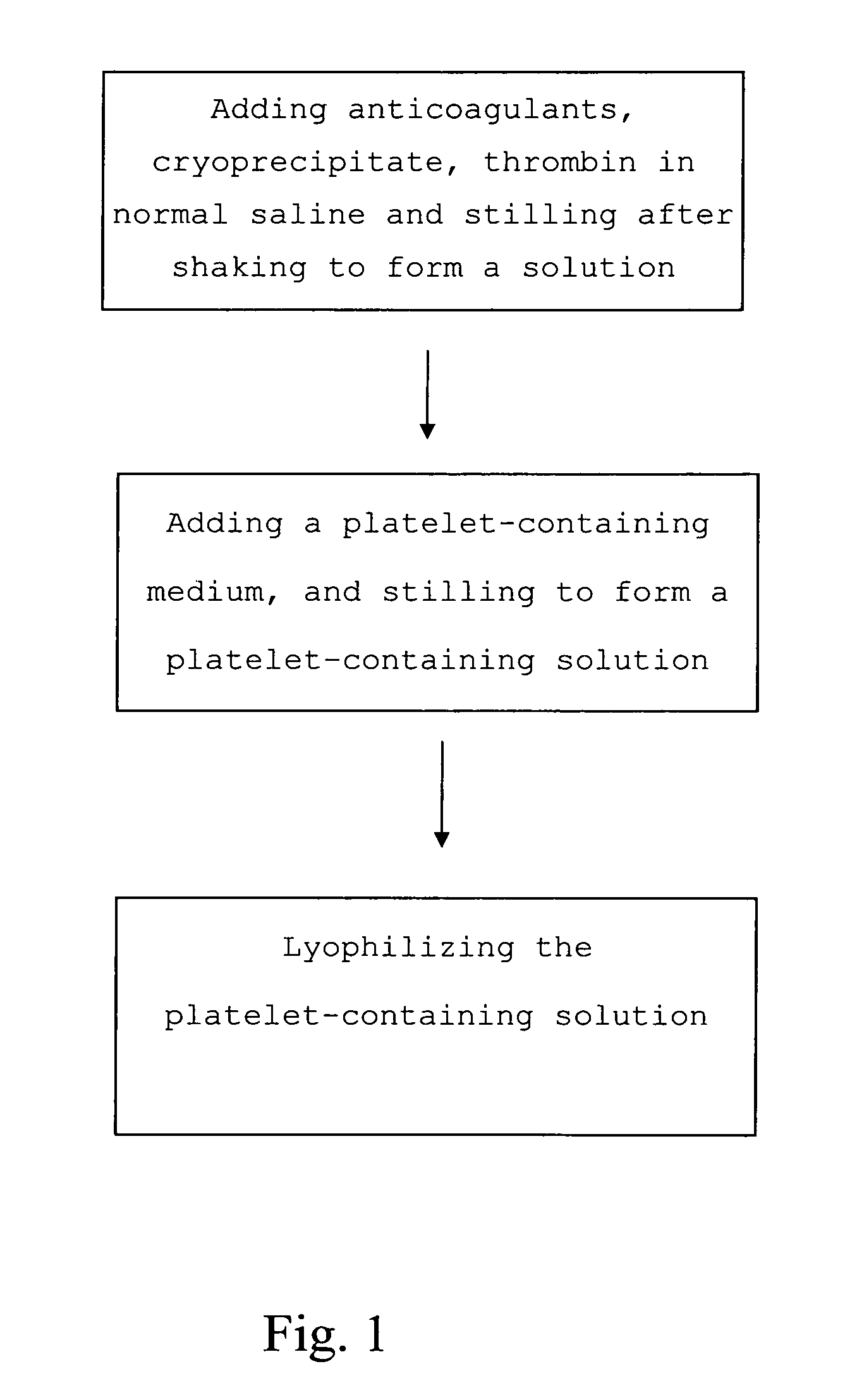
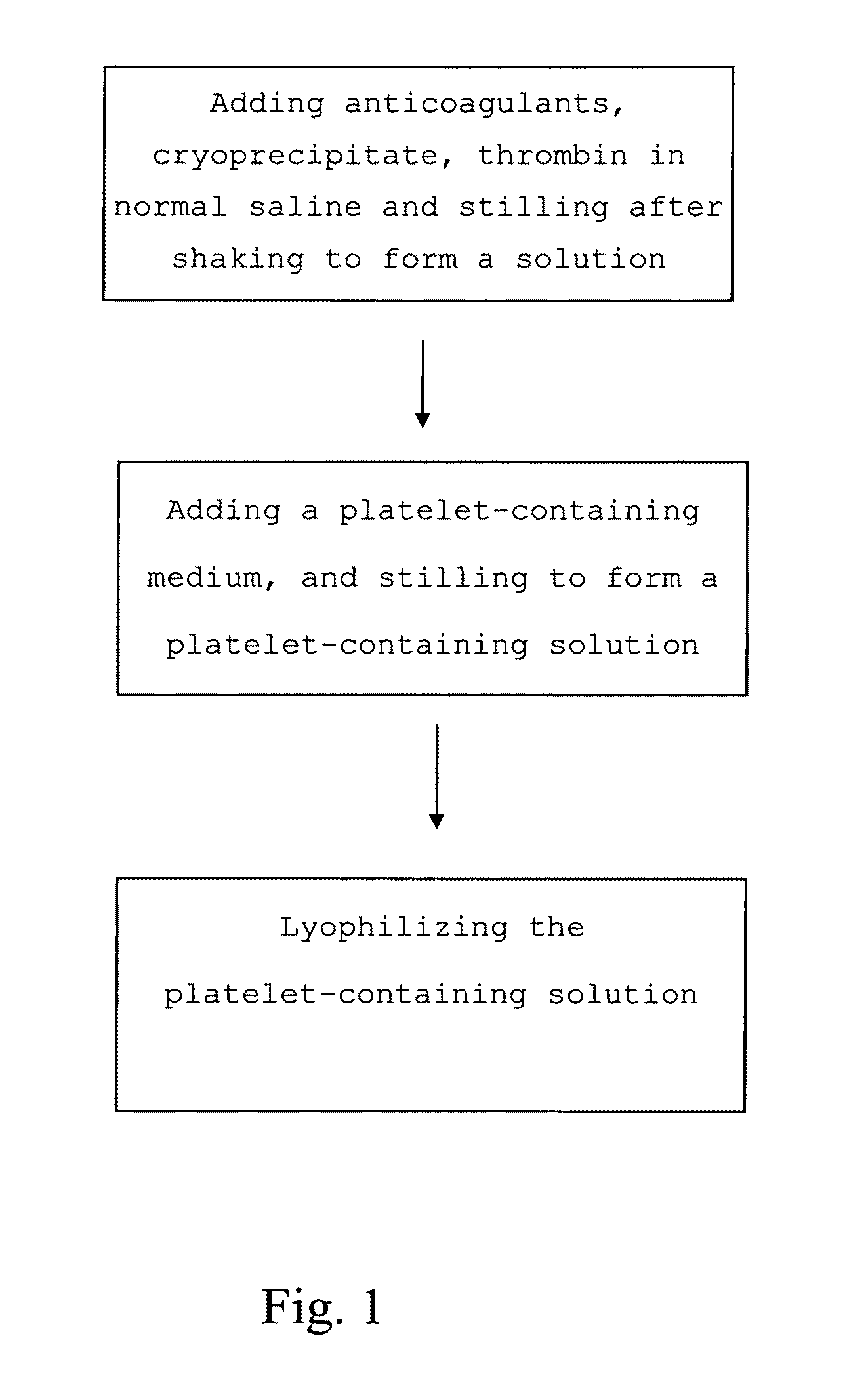
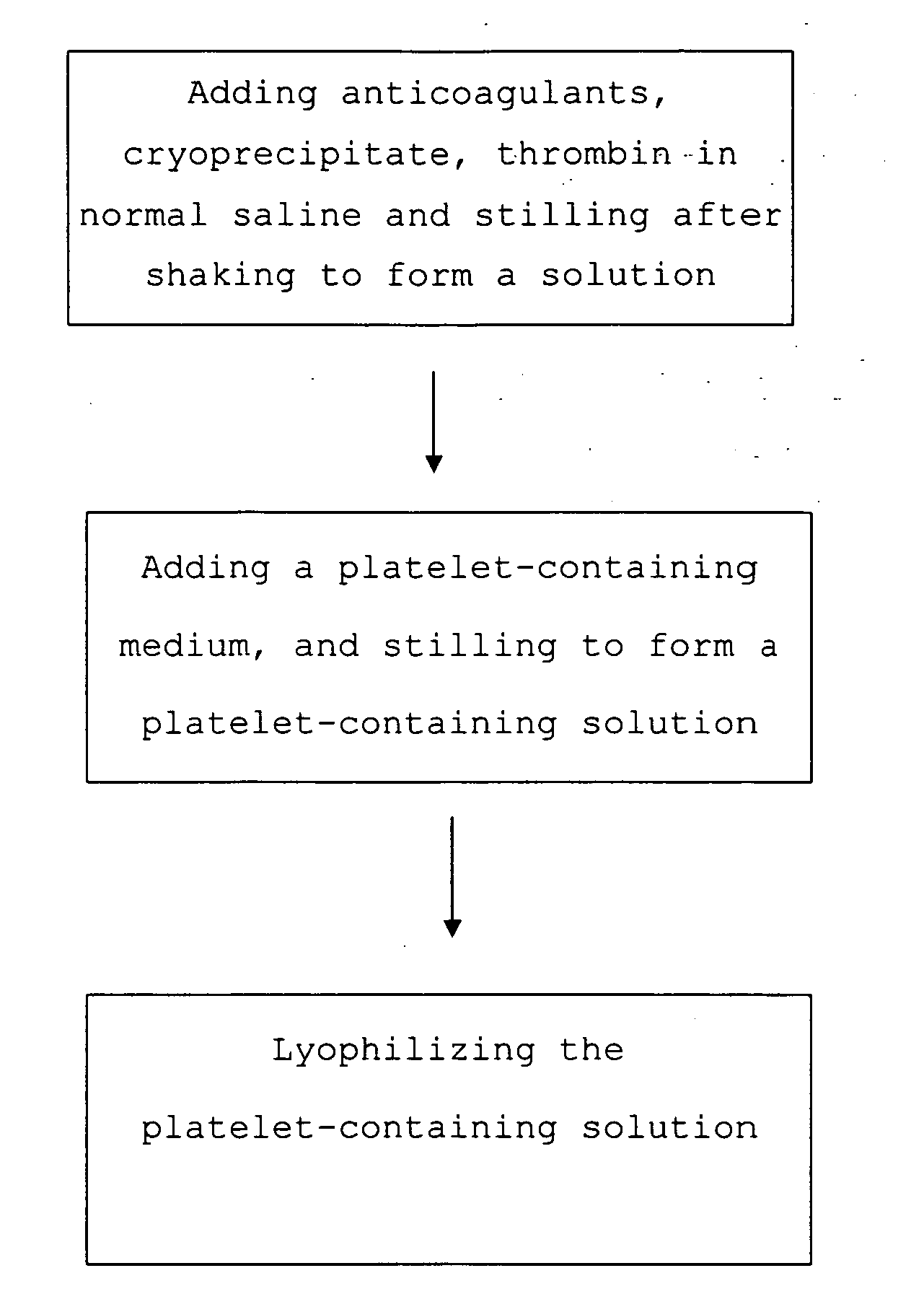
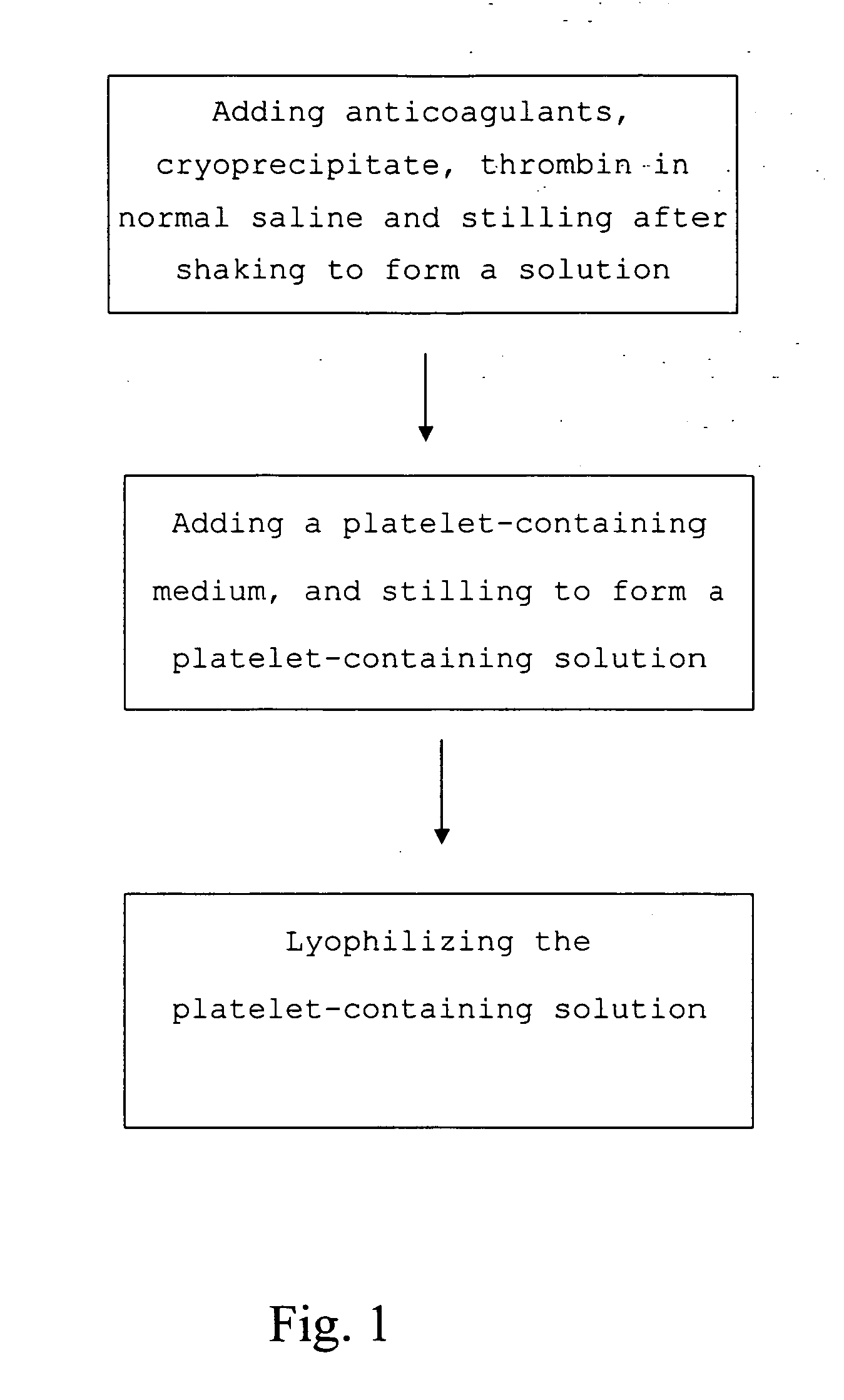
The present invention provides a platelet-containing composition prepared by contacting platelets with a medium for preserving. The medium comprises anticoagulant, cryoprecipitate and thrombin. The present invention also provides a method for long-term preservation of platelets, comprising the steps of: (a) adding an anticoagulant, cryoprecipitate, thrombin in normal saline; (b) adding a platelet-containing medium into the solution formed in step (a); and (c) lyophilizing the platelet-containing solution formed in step (b). Moreover, the present invention yet provides a medium for preserving non-nucleus cells.
Owner:LIN CHENG CHIH +1
Polyvinyl Pyrollidone Cryoprecipitate Extraction of Clotting Factors
InactiveUS20080281081A1Increase productionEliminate and suppressFactor VIISurgical adhesivesBlood collectionCITRATE ESTER
Blood collection, processing and transfer leads to the separation of discrete fractions by adding additional citrate (trisodium citrate) to bring the citrate concentration to 10%-15% w / v thereby leading to enhanced yield and purity of cryoprecipitate. The improved cryoprecipitate then yields concentrated clotting factors by an improved extraction process which uses polyvinyl pyrollidone to reduce the extraction of fibrinogen. Following extraction the remaining cryoprecipitate can advantageously be formed into a fibrin fabric used in surgeries and in the treatment of wounds
Owner:SHANBROM TECH
Method for stabilizing a cryoprecipitate of plasmatic proteins for being subjected to a viral inactivation thermal treatment
ActiveUS7727743B2Improve protectionImprove solubilityPowder deliveryPeptide/protein ingredientsProtein compositionCryoprecipitate
The invention relates to a method for obtaining cryoprecipitatable proteins, comprising a viral inactivation step by thermally treating a lyophilisate of these proteins, comprising, before rendering the proteins in the form of a lyophilisate, an initial addition step, to these proteins, of a stabilizing and solubilizing formulation containing a mixture consisting of arginine, at least one hydrophobic amino acid and of tribasic sodium citrate. The invention also relates to a concentrate consisting of at least one cryoprecipitable protein containing the stabilizing and solubilizing formulation introduced according to the method and being suited for therapeutic use.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
Method for preparing cryoprecipitate and method for preparing blood coagulation factor VIII preparation by using cryoprecipitate
ActiveCN103848886AReduce the amount of solutionFactor VIIPeptide preparation methodsBlood coagulation factor VIIICryoprecipitate
The invention discloses a method for preparing a cryoprecipitate. The method comprises the steps of (1) thawing: selecting fresh frozen plasma, and heating to obtain thawed plasma of which the temperature is 0-5 DEG C; (2) filtering: filtering under the condition that the temperature of the thawed plasma is 0-5 DEG C to obtain filtrate and filter residues; (3) centrifuging: centrifuging under the condition that the temperature of the filtrate is 0-5 DEG C to obtain a precipitate; (4) combining the filter residues obtained in step (2) and the precipitate obtained in step (3) to obtain the cryoprecipitate. The preparation method disclosed by the invention is simple, and the prepared cryoprecipitate is high in yield and human blood coagulation factor VIII content, so the preparation method has a good industrial application prospect.
Owner:CHENGDU RONGSHENG PHARMA
Process for extracting human coagulation factor VIII from plasma
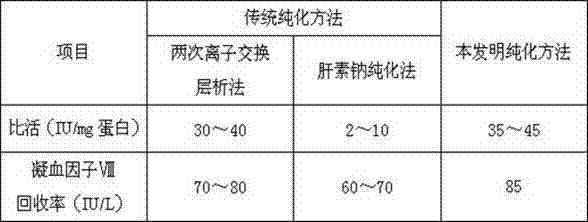
The invention discloses an economic and simple method for improving the separation efficiency, activity, specific activity and recovery rate of coagulation factor VIII. The method comprises washing cryoprecipitate twice with sodium heparin firstly, then carrying out ion exchange chromatography and eluting with an eluent to obtain higher-purity coagulation factor VIII. Compared with traditional methods, the method has the advantages of simple process and low cost; the separation efficiency, activity, specific activity and recovery rate of the coagulation factor VIII are also improved.
Owner:新疆德源生物工程有限公司
Solubilization technology for producing human fibrinogen
ActiveCN103405754AImprove utilizationEffective inactivationPowder deliveryFibrinogenSolubilityLipid formation
The invention relates to a solubilization technology for producing human fibrinogen. The solubilization technology comprises following main steps: (1) source plasma treatment; (2) dissolving and centrifuging of cryoprecipitate; (3) inactivation of viruses; (4) chromatography purification; (5) centrifugal separation; (6) dissolving of sediments; (7) degerming and filtering; (8) split charging and freeze drying. The solubilization technology has the advantages that the purity of the extracted product can be up to over 95%; the content of foreign protein is low; a lipid envelope virus and a non-lipid envelope virus can be effectively activated; the safety of the product is ensured; arginine hydrochloride and glycine are adopted as stabilizers; the product can be fully protected in a freeze-drying process; the temperature change of the product in the freeze-drying process is stable by prolonging the freeze-drying time (about 4-8 days, and about 3 days for general factories); a freeze-dried product with a uniform structure can be obtained; the solubility of the human fibrinogen is increased; the product is quick to dissolve after being re-dissolved; no highly visible denatured protein is generated.
Owner:WUHAN ZHONGYUAN RUIDE BIOLOGICAL PROD CO LTD
Preparation method of human VIII blood coagulation factor
ActiveCN103613658AEasy to manufactureQuality is easy to controlFactor VIIPeptide preparation methodsFreeze-dryingDissolution
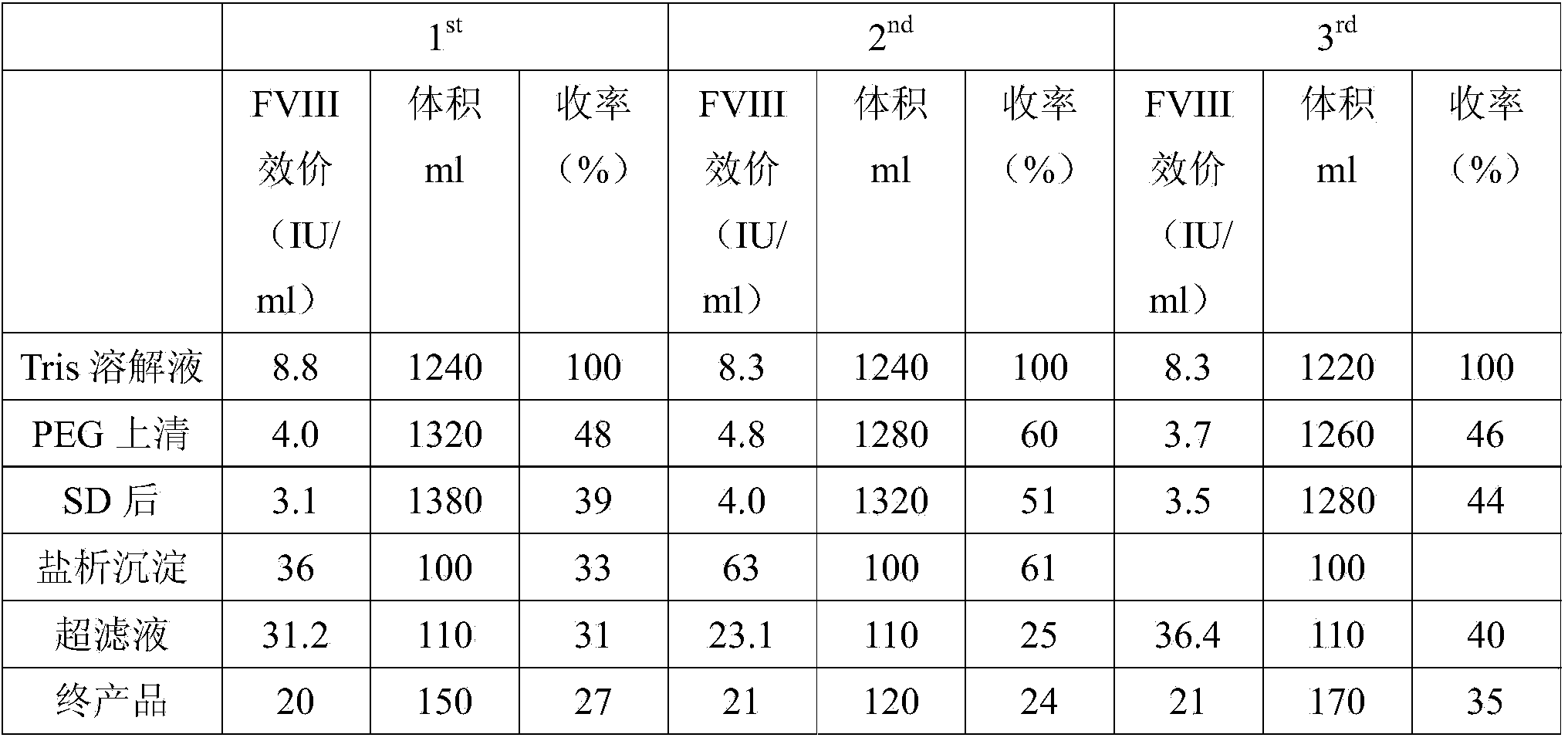
The invention discloses a preparation method of a human VIII blood coagulation factor. The preparation method comprises the following steps: (1) separating cryoprecipitates from human plasma, and adding a Tris-HCl dissolving solution to dissolve; (2) primary PEG (Polyethylene Glycol) precipitation: adding a PEG 1 solution into the dissolving solution, stirring, standing, and then, centrifuging; (3) collecting a centrifuged suspension liquid, and adding an S / D solution to inactivate; (4) secondary PEG precipitation: clarifying the inactivated suspension liquid, filtering to obtain a filtrate, transferring the filtrate into a low-temperature reaction tank, adding a PEG 2 solution under the condition of stirring, stirring, standing, and then, centrifuging; and (5) cleaning a precipitate, dissolving the sediment by using the dissolving solution, centrifuging after complete dissolution, collecting the suspension liquid, filtering, preparing, degerming, split charging, freeze-drying, capping, and carrying out dry heat inactivation to obtain the human VIII blood coagulation factor. A two-step PEG precipitation method is adopted in the method disclosed by the invention, so that the production process is simplified, and the requirement for large equipment is reduced; the preparation method is small in floor area, simple in operation, low in cost and capable of increasing the labor efficiency and economic benefit.
Owner:TONROL BIOLOGICAL PHARM CO LTD
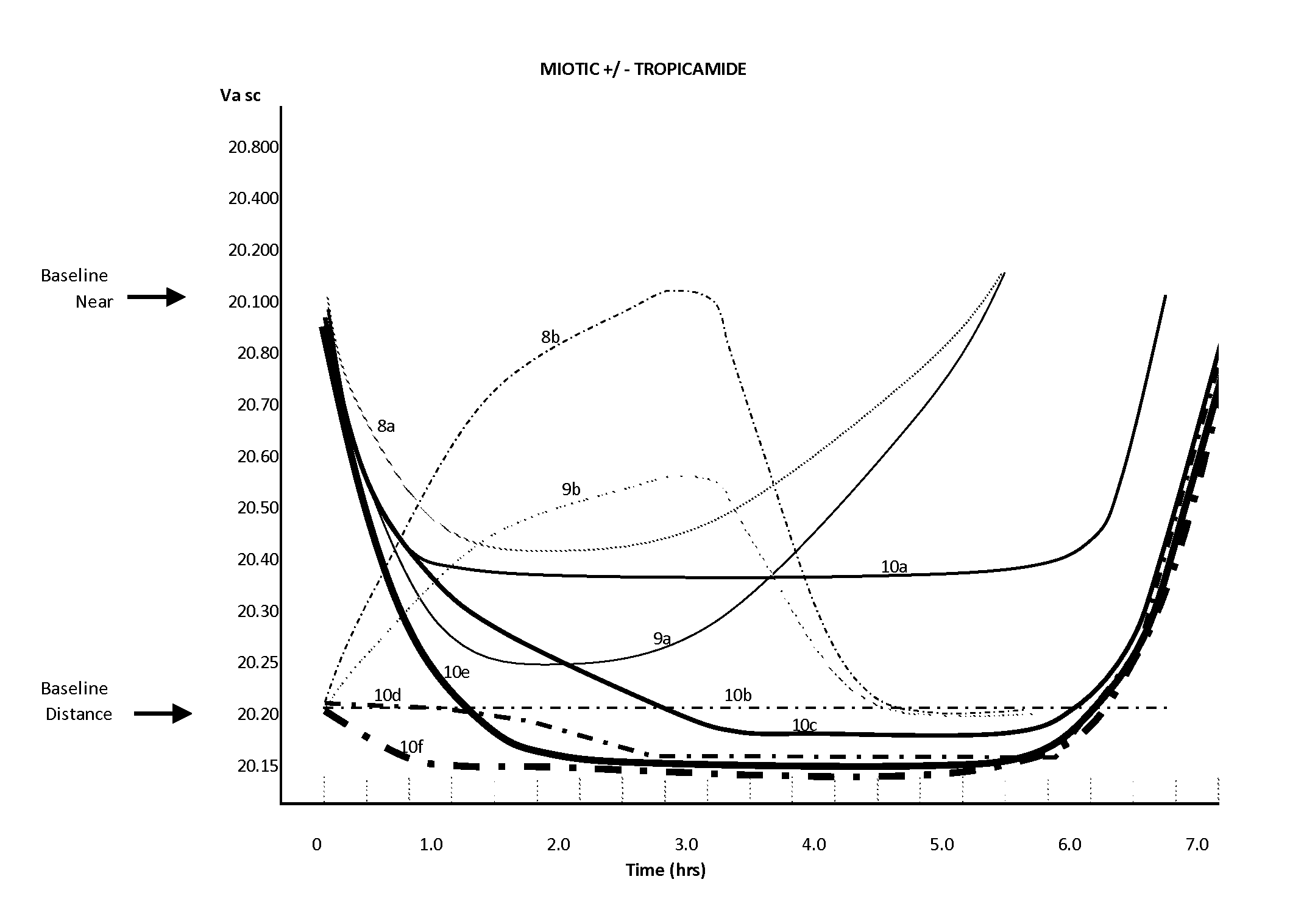
Compositions and Methods for the Improvement of Distance Vision and the Treatment of Refractive Errors of the Eye
ActiveUS20150290126A1Eliminating optical aberrationImprove visual acuityBiocideInorganic non-active ingredientsRefractive errorCryoprecipitate
The invention provides compositions and methods for the improvement of distance vision. The invention further provides compositions and methods for the treatment of refractive errors of the eye. The invention further provides compositions that preferably comprise aceclidine lyophilized with a cryoprecipitate separate or together with a diluent comprising a cycloplegic agent, a surfactant, and optionally a viscosity enhancer.
Owner:LENZ THERAPEUTICS INC
Preparation method of human fibrinogen
InactiveCN105504046AEasy extractionImprove utilizationFibrinogenPeptide preparation methodsFiberFiltration
The invention discloses a preparation method of human fibrinogen. The method comprises the following steps that 1, cryoprecipitate serves as a staring material and is dissolved; 2, clarification and filtration are conducted; 3, solvent / detergent (S / D) inactivation is conducted; 4, Q Sepharose fastflow gel adsorption chromatographic processing is conducted; 5, centrifugation is conducted, precipitation is collected, sterilizing, subpackaging, freeze-drying, capping and dry-heating inactivating are conducted, and the product is obtained. The preparation method of the human fibrinogen is simple, easy to operate, low in cost and capable of increasing the comprehensive utilization ratio of blood plasma, and the obtained product is high in purity, activity and safety.
Owner:TONROL BIOLOGICAL PHARM CO LTD
Compositions and methods for the improvement of distance vision and the treatment of refractive errors of the eye
ActiveUS9314427B2Organic active ingredientsPharmaceutical delivery mechanismRefractive errorCryoprecipitate
The invention provides compositions and methods for the improvement of distance vision. The invention further provides compositions and methods for the treatment of refractive errors of the eye. The invention further provides compositions that preferably comprise aceclidine lyophilized with a cryoprecipitate separate or together with a diluent comprising a cycloplegic agent, a surfactant, and optionally a viscosity enhancer.
Owner:LENZ THERAPEUTICS INC
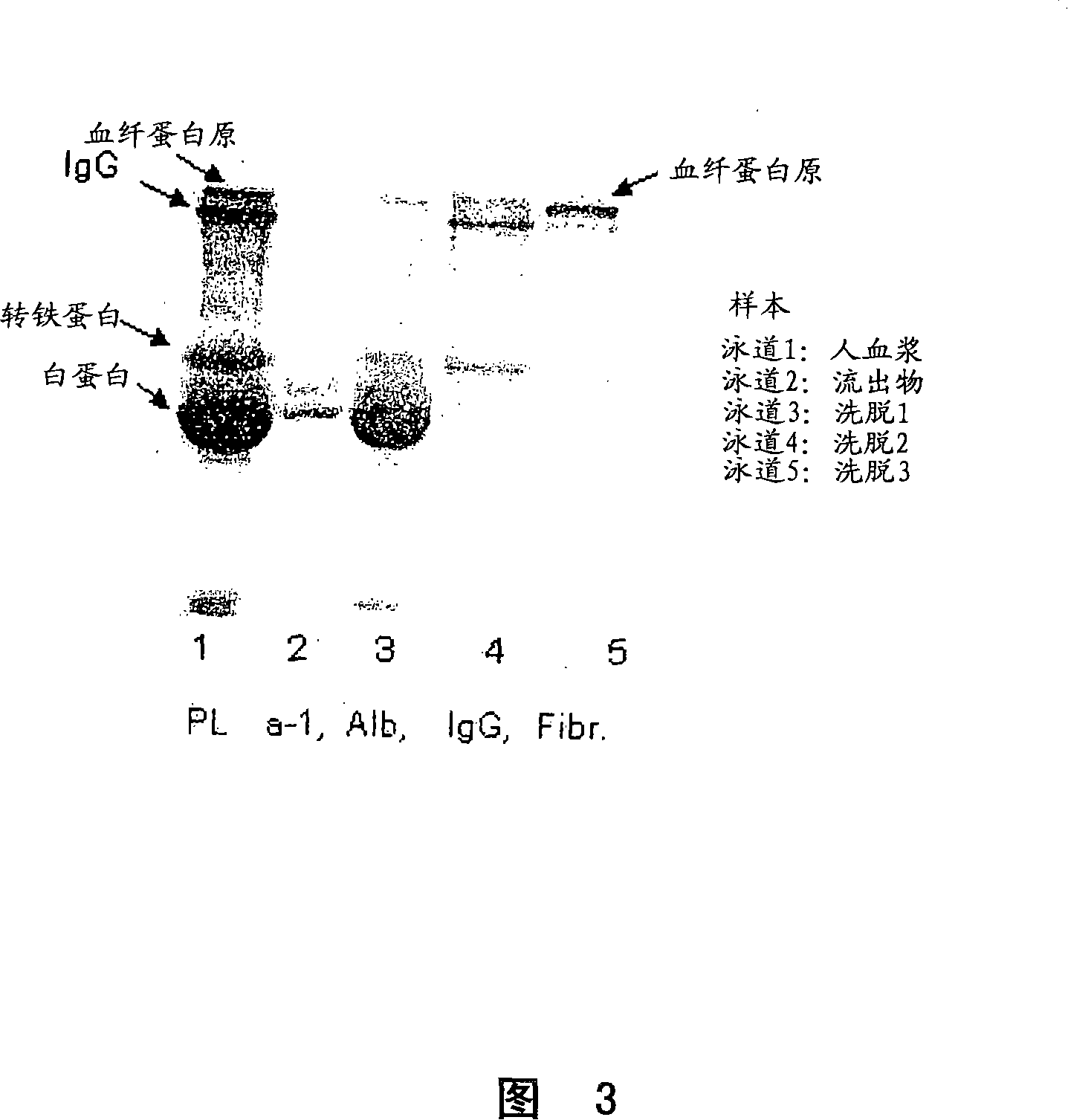
Process for protein isolation
In one aspect, the present invention is directed to a process for isolating proteins from a solution comprising proteins, wherein the solution is selected from the group consisting of: crude bood plasma, blood serum, cryosupernatant derived from plasma, fractionated human plasma, cryoprecipitate derived from plasma and recombinant broths. The process involves providing a solid separation medium having the formula: M-S-L wherein M is a matrix backbone, S is an optional spacer arm, and L is a ligand which is mercaptonicotinic acid, contacting the solid separation medium with the solution comprising the proteins, such that at least one of the proteins becomes reversibly bound bound to said solid separation medium. At least one elution step is then performed to selectively elute at least one protein fraction from the solid separation medium. In another aspect, the present invention is directed to a process for isolating Factor VIII and / or Factor IX.
Owner:AVT PLASMA
Preparation method of human blood coagulation factor VIII and human blood coagulation factor VIII product
ActiveCN107880112AHigh recovery rateTaller than aliveFactor VIIPeptide preparation methodsWhole blood productIon chromatography
The invention discloses a preparation method of a human blood coagulation factor VIII and a human blood coagulation factor VIII product and relates to the field of blood products. The preparation method of the human blood coagulation factor VIII comprises the following steps: dissolving a cryoprecipitate with a 0.015-0.025mol / L tromethamine solution in a mass ratio of (3-5): 1 by reasonably processing the cryoprecipitate; and carrying out separation and purification by means of a polyethylene glycol precipitating method combined with ion exchange chromatography, wherein the recovery ratio of the human blood coagulation factor VIII and the specific activity of a final product can be improved effectively, the yield reaches 180-240IU / L plasma, and the specific activity is not lower than 100IU / mg proteins. The prepared human blood coagulation factor VIII product is rich in vWF factors and the proportion of the vWF factors and the human blood coagulation factor VIII is close to 1: 1. Besides treating hemophiliac A, the human blood coagulation factor VIII can be also used for treating patients with angiohemophilia, and the human blood coagulation factor VIII has good stability and heat resistance.
Owner:HUALAN BIOLOGICAL ENG INC +2
Medium and method for preserving platelets, red blood cells, and other non-nucleus cells and platelets-containing composition
The present invention provides a platelet-containing composition prepared by contacting platelets with a medium for preserving. The medium comprises anticoagulant, cryoprecipitate and thrombin. The present invention also provides a method for long-term preservation of platelets, comprising the steps of: (a) adding an anticoagulant, cryoprecipitate, thrombin in normal saline; (b) adding a platelet-containing medium into the solution formed in step (a); and (c) lyophilizing the platelet-containing solution formed in step (b). Moreover, the present invention yet provides a medium for preserving non-nucleus cells.
Owner:LIN CHENG CHIH +1
Compositions suitable for treatment of spinal disease, disorder or condition
InactiveUS8968724B2Raise the ratioIncreased proliferationBiocideNervous disorderDiseaseCryoprecipitate
Owner:OMRIX BIOPHARM
Method for Chromatography
ActiveUS20180127459A1High purityHigh activityFactor VIICation exchanger materialsCryoprecipitateFactor VIII vWF
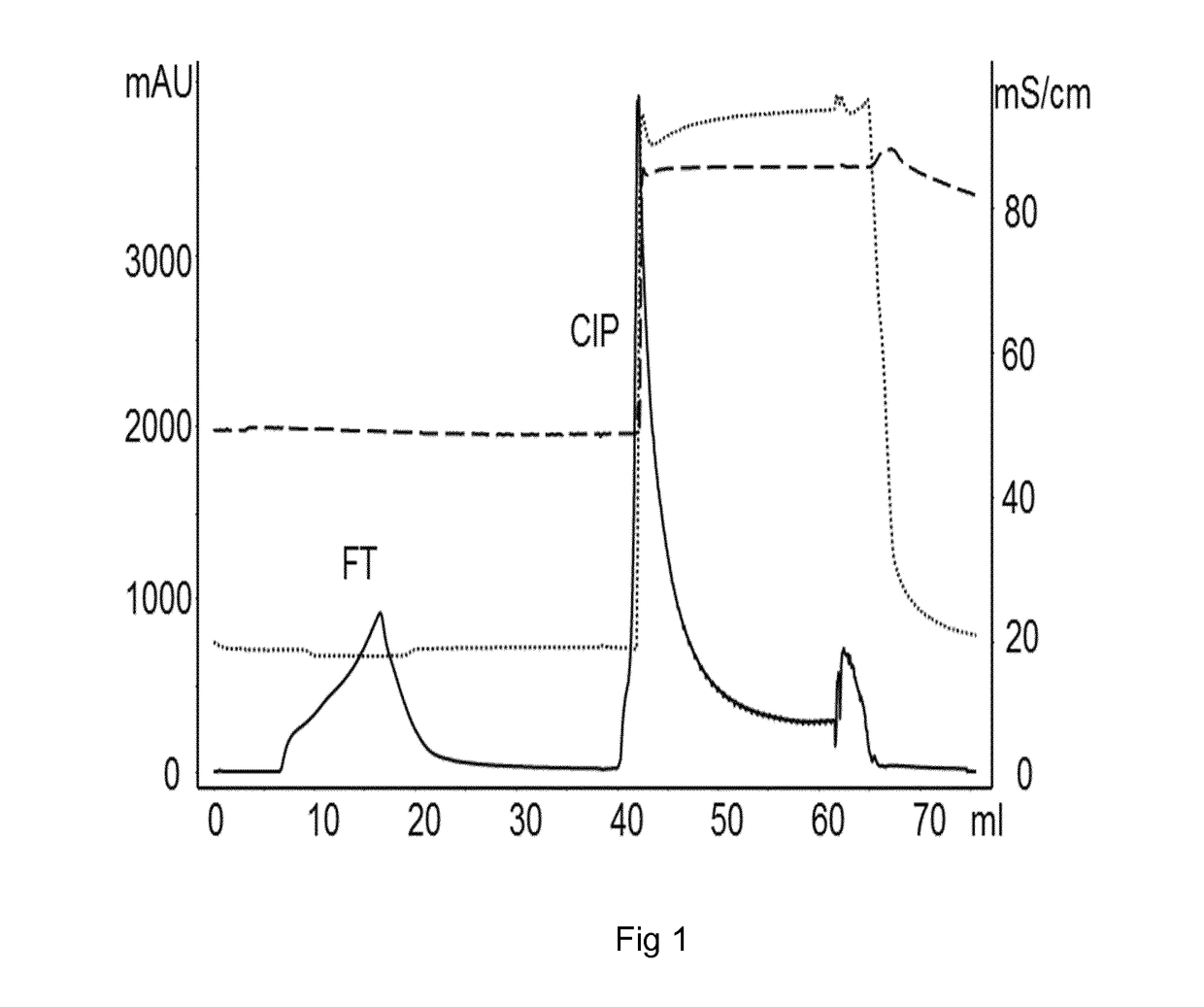
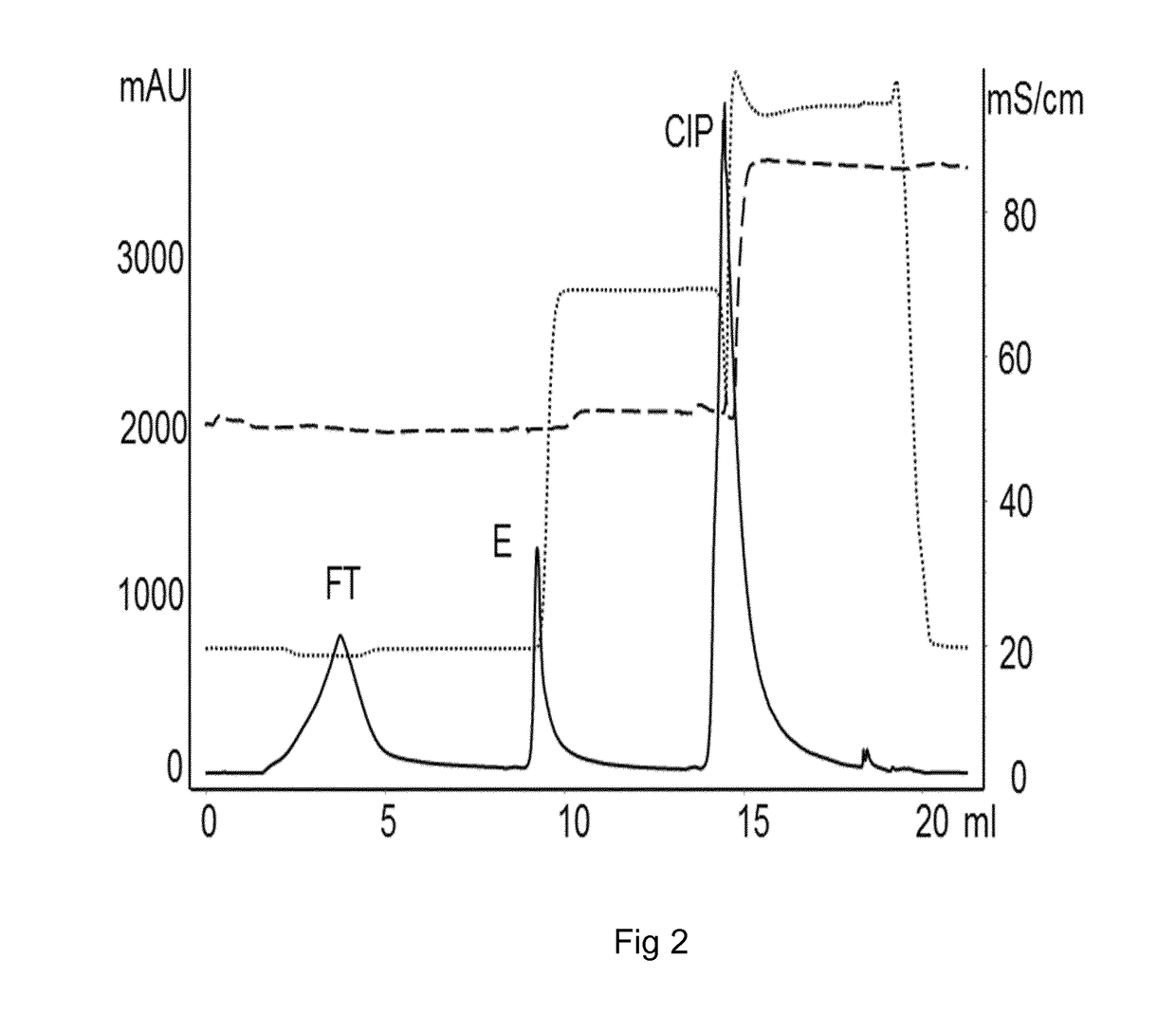
The present invention relates to the field of chromatography. More closely, the invention relates to a chromatographic method for purification of Factor VIII and von Willebrand factor from a cryoprecipitate of plasma. The chromatographic method is performed on a matrix comprising an inner porous core and outer porous lid surrounding said core.
Owner:CYTIVA BIOPROCESS R&D AB
Method for preparing cryoprecipitate coagulation factor
InactiveCN107281222AShort preparation timeAccurate capacityPharmaceutical delivery mechanismMammal material medical ingredientsCryoprecipitateFrequency conversion
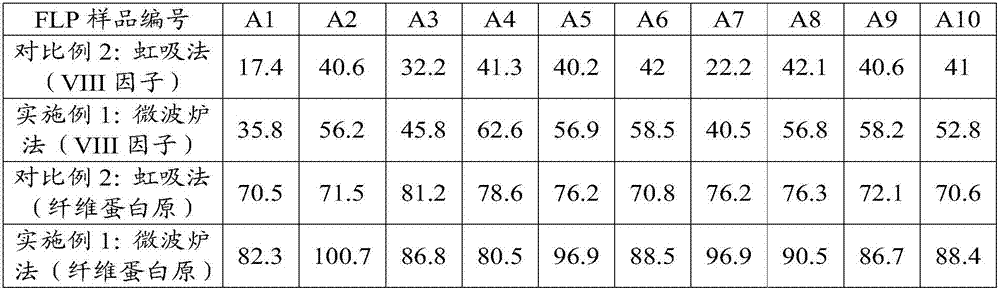
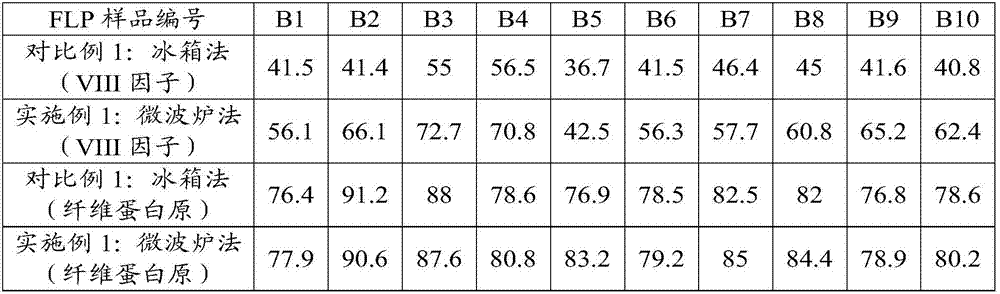
The invention provides a method for preparing a cryoprecipitate coagulation factor. The method for preparing the cryoprecipitate coagulation factor, provided by the invention, comprises the following steps: a) providing fresh lyophilized plasma; b) unfreezing: unfreezing the fresh lyophilized plasma through microwaves by adopting a frequency conversion microwave heating device to unfreezing temperature, so as to obtain an ice-block-shaped mixture; c) separating: separating a precipitated substance, which is not dissolved coldly, from the ice-block-shaped mixture, so as to obtain the cryoprecipitate coagulation factor. The method provided by the invention has the advantages of simple equipment and convenience for operation, short preparation time and accurate preparation capacity, more content of obtained VIII factors, good clinical transfusion effect and the like.
Owner:侯广安 +3
Method for liquid preservation of intermediate product of human prothrombin complex
ActiveCN109593747AReduce stabilityReduce the risk of contaminationEnzyme stabilisationPeptidasesCryoprecipitateLiquid state
The invention relates to a method for liquid preservation of an intermediate product of a human prothrombin complex. The method comprises the following steps: (1) carrying out plasma adsorption, namely stirring and adsorbing plasma of which cryoprecipitate is removed by using balanced gel; (2) washing the gel, and removing protein components in a non-human prothrombin complex; (3) eluting the gel,and collecting an elution liquid, namely eluting the gel washed in the step (2) by using an elution liquid, and collecting the elution liquid; (4) carrying out ultrafiltration on the elution liquid;(5) preserving an intermediate product, namely sealing and preserving a concentrated liquid at a non-frozen state of the intermediate product; (6) carrying out detection. By adopting the method, the intermediate product of the human prothrombin complex is preserved, and good activity of the intermediate product can be maintained; control on the production process is facilitated, and the risks of instability and contamination of a product at switched solid and liquid states can be reduced; cryopreservation and remelting processes are avoided, and the production efficiency can be improved.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
Medium and method for preserving platelets, red blood cells, and other non-nucleus cells and platelets-containing composition
InactiveUS20050287516A1For long-term storageDead animal preservationBlood disorderCryoprecipitateThrombin activity
The present invention provides a platelet-containing composition prepared by contacting platelets with a medium for preserving. The medium comprises anticoagulant, cryoprecipitate and thrombin. The present invention also provides a method for long-term preservation of platelets, comprising the steps of: (a) adding anticoagulants, cryoprecipitate, thrombin in normal saline; (b) adding a platelet-containing medium into the solution formed in step (a); and(c) lyophilizing the platelet-containing solution formed in step (b). Moreover, the present invention yet provides a medium for preserving non-nucleus cells.
Owner:SU CHENG YAO +1
Production method of human fibrinogen
ActiveCN101703763BEffective inactivationEnsure safetyPowder deliveryPeptide/protein ingredientsWater bathsFiber
The invention provides a production method of human fibrinogen, which is against the method that domestic conventional manufacturers extract and separate human fibrinogen from component I, and the method for extracting fibrinogen from cryoprecipitate is adopted; an S / D method and a water-bath heat treating method are adopted to carry out double-virus inactivation, thereby effectively inactivatingfat enveloped virus and non-fat enveloped virus, and ensuring the security of products; the purity of extracted products reaches as high as more than 90%, the protein content of impurities is low, and the clinical side reaction of the product is small. In the production process of the human fibrinogen of the invention, glycine is utilized as a stabilizer, the freeze-drying time is prolonged about6-8 days to ensure the temperature variation of the product to be stable in freeze-dying process, but the freeze-drying time of general manufacturers is about 3 days; after being freeze-dried for 6-8days, the product has uniform structure, then water bath heat treatment is carried out on the product, thus the product can be evenly heated in the shortest time, thereby achieving the purposes of effectively inactivating DNA and RNA non-fat enveloped virus and simultaneously ensuring the stability of human fibrinogen.
Owner:GREEN CROSS CHINA BIOLOGICAL PRODS
Pre-purifying method for cryoprecipitate
InactiveCN105175483AImprove the purification effectTaller than aliveFactor VIIPeptide preparation methodsCryoprecipitatePolyethylene glycol
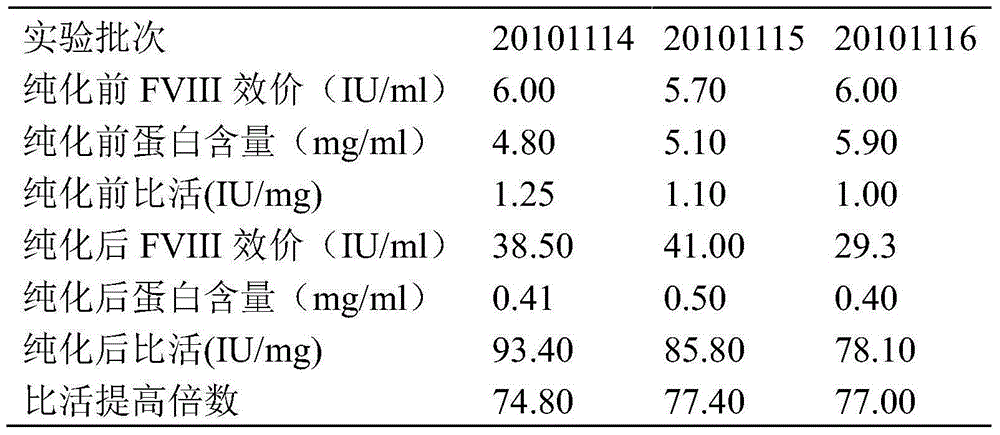
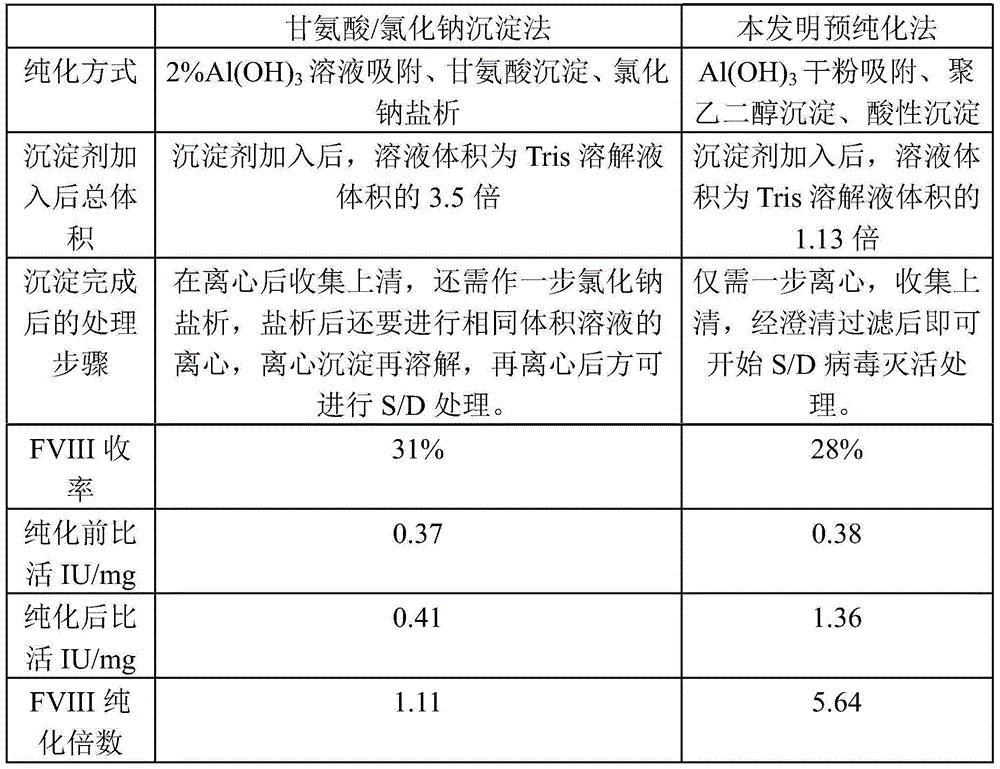
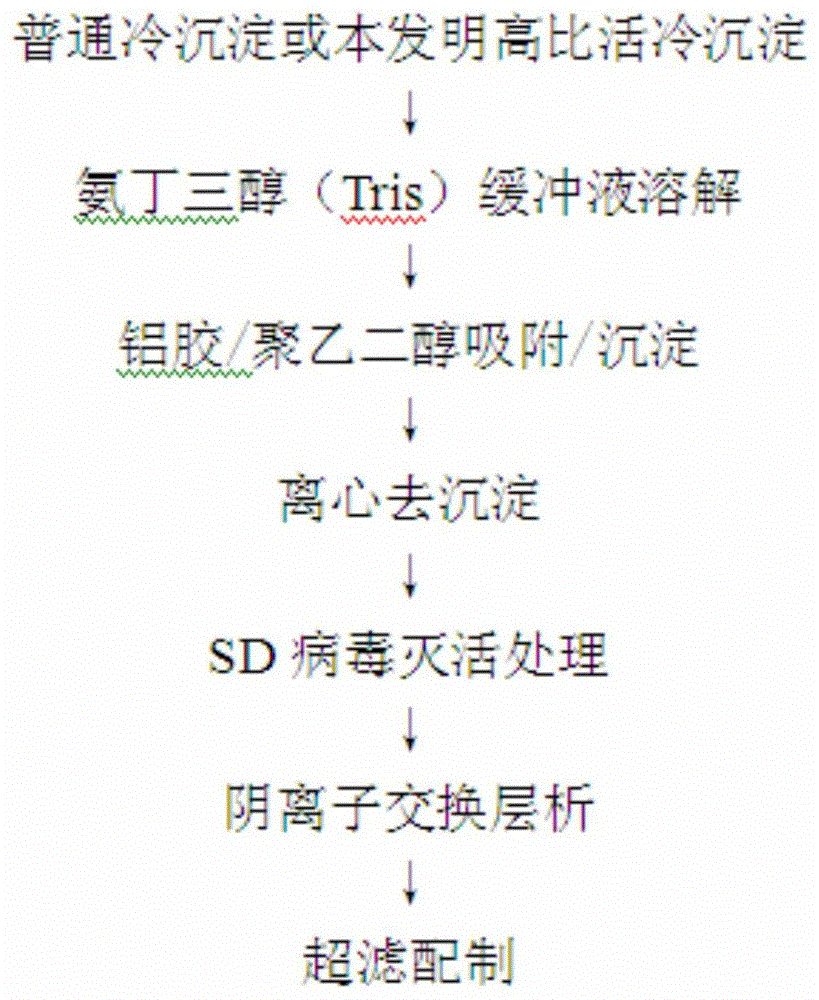
The invention discloses a pre-purifying method for a cryoprecipitate. The method comprises the following steps that 1, the cryoprecipitate is taken and dissolved; 2, aluminum hydroxide gel dry powder is added, the using amount of the aluminum hydroxide gel dry powder accounts for 5-30%w / w of that of the cryoprecipitate, and stirring is performed for 15-30 min; 3, polyethylene glycol is added until the final concentration of the polyethylene glycol is 3.0-4.0%w / v, the mixture is evenly mixed, the pH is regulated to 6.0-6.5, and stirring is continuously performed for 15-60 min; 4, centrifuging is performed for 10-30 min to obtain supernatant, filtering is performed with a filter membrane, and then the method is completed. According to the pre-purifying method, the purifying effect is good, the FVIII specific activity is increased by 3.69-5.64 times, operation is easy, and the quality is controllable.
Owner:CHENGDU RONGSHENG PHARMA
A kind of method and application thereof for preparing cryoprecipitate
ActiveCN104086620BTaller than aliveFactor VIIPeptide preparation methodsComparative testCentrifugation
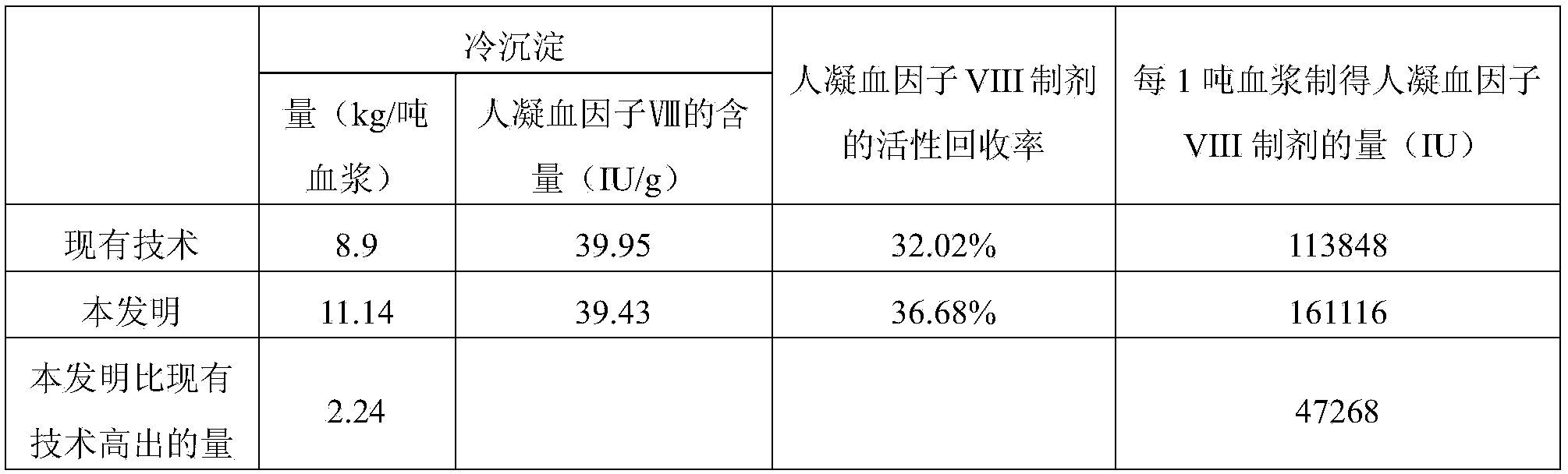
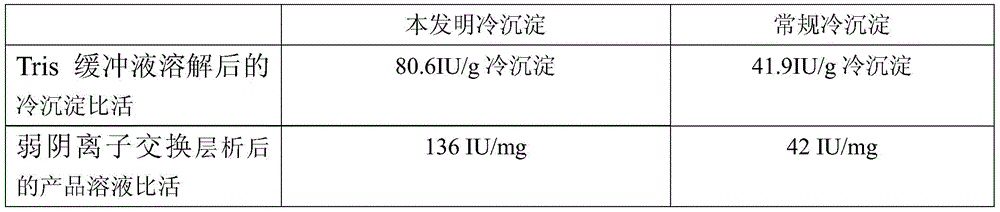
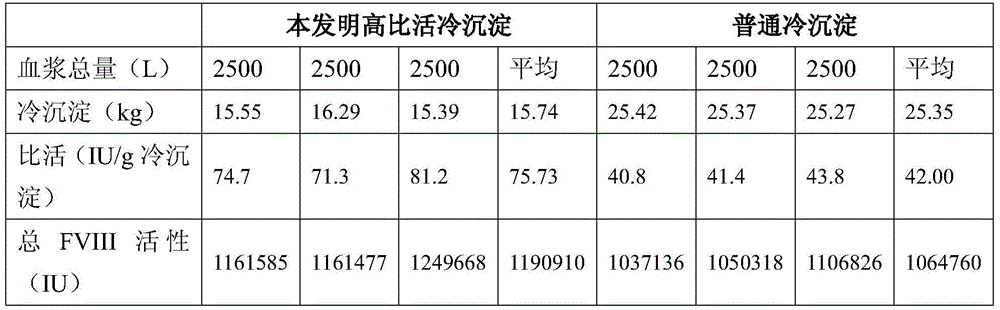
The invention provides a cryoprecipitate preparation method comprising the following steps: (1) healthy human plasma cryopreserved at a temperature of -20 DEG C is fetched; the temperature of the plasma is increased to -15 DEG C to -5 DEG C within 24h; (2) under a circulation water condition with a temperature no higher than 37 DEG C, the plasma is completely molten; (3) the temperature of the plasma is controlled at -2 DEG C to 5 DEG C, and the plasma is allowed to stand for 3-9h; and (4) centrifugation is carried out, such that cryoprecipitate is obtained. As a result of comparative tests, it is proved that human coagulation factor VIII prepared with the cryoprecipitate provided by the invention has a specific activity approximately 4 times as much as that of human coagulation factor VIII with a common cryoprecipitate as a raw material. The specific activity is much higher than a standard of 1IU / mg specified by Chinese Pharmacopoeia. It is known in the art that human coagulation factor VIII is expensive. With the greatly increased specific activity, economic benefit can be greatly improved. The method has good industrial application prospect.
Owner:CHENGDU RONGSHENG PHARMA
A preparation process for extracting human fibrinogen from the waste material of coagulation factor ⅷ extracted by cryoprecipitation
ActiveCN104231072BSave scarce plasma resourcesFactor VIIFibrinogenBlood coagulation factor VIIIEthanol precipitation
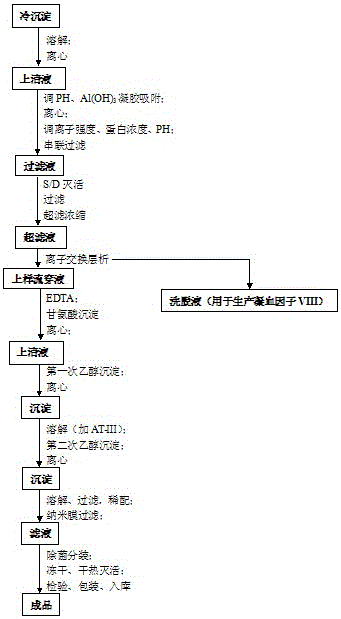
The invention discloses a preparation process for extracting human fibrinogens from waste for extracting cryoprecipitated blood coagulation factor VIII. The preparation process is characterized by comprising the steps of cryoprecipitation dissolution, 2% aluminium hydroxide gel absorption, ion strength adjustment, series connection filtering, S / D viral inactivation, ion-exchange chromatography, EDTA Ca2+ removal, glycine precipitation, primary low-temperature ethanol precipitation, AT-III thrombin inhibition, secondary low-temperature ethanol precipitation, nanofilm filtering and dry-heat inactivation. For ensuring the safety, a nanofilm ia added to filter virus except S / D and dry-heat inactivation. By means of an added AT-III inactivated thrombin and EDTA Ca2+ removal process, fibrinogen in the production process is effectively prevented from being activated into fibrous protein. The glycine precipitation is utilized to remove fibrous protein monomers and polymers in products so as obtain high-purity human fibrinogen. The preparation products are safe and reliable, redissolution time is short, the clinic first-aid demand is met, and meanwhile the preparation process has important significance on indirect saving of scarce plasma resources.
Owner:华润博雅生物制药集团股份有限公司
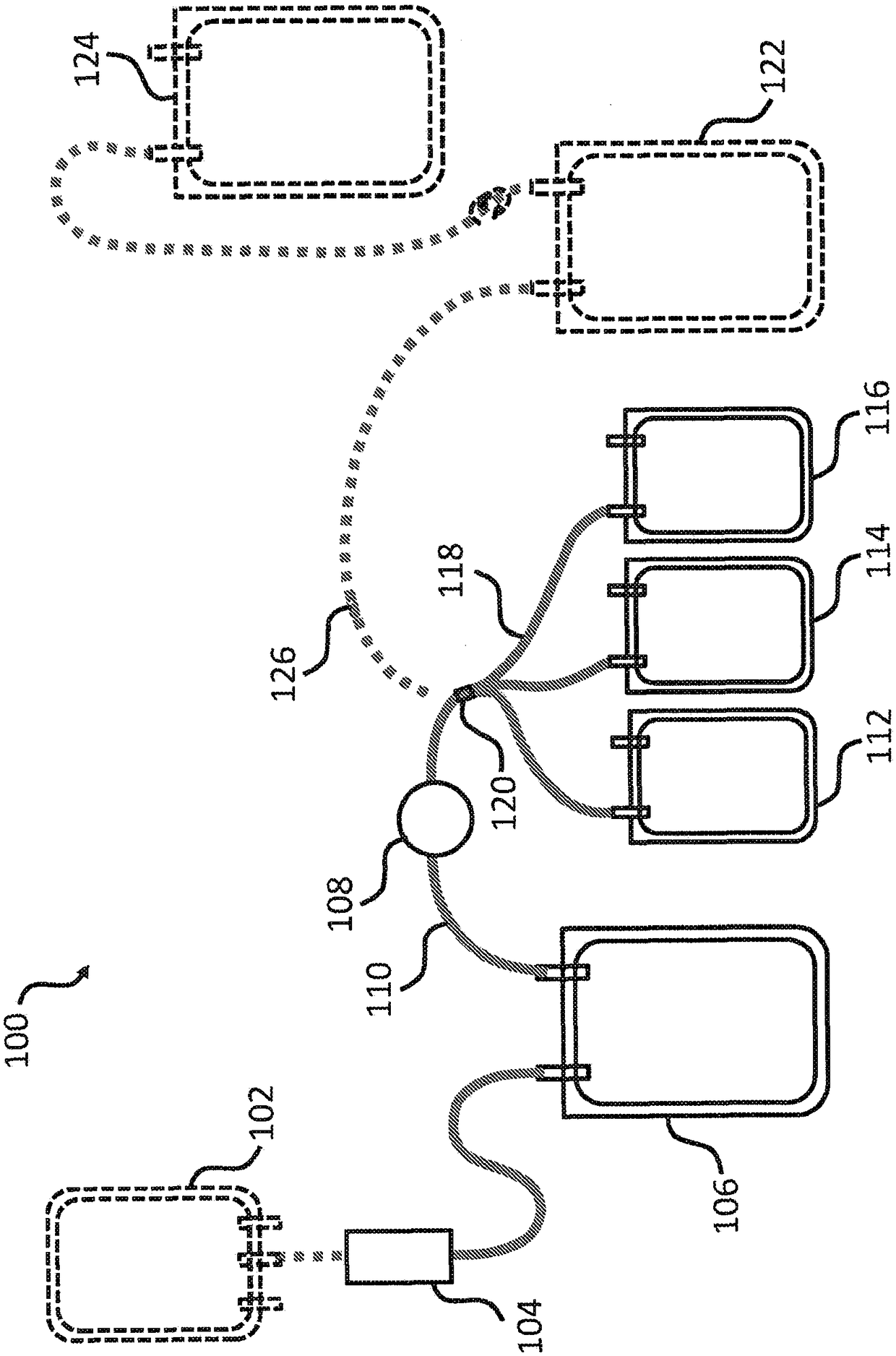
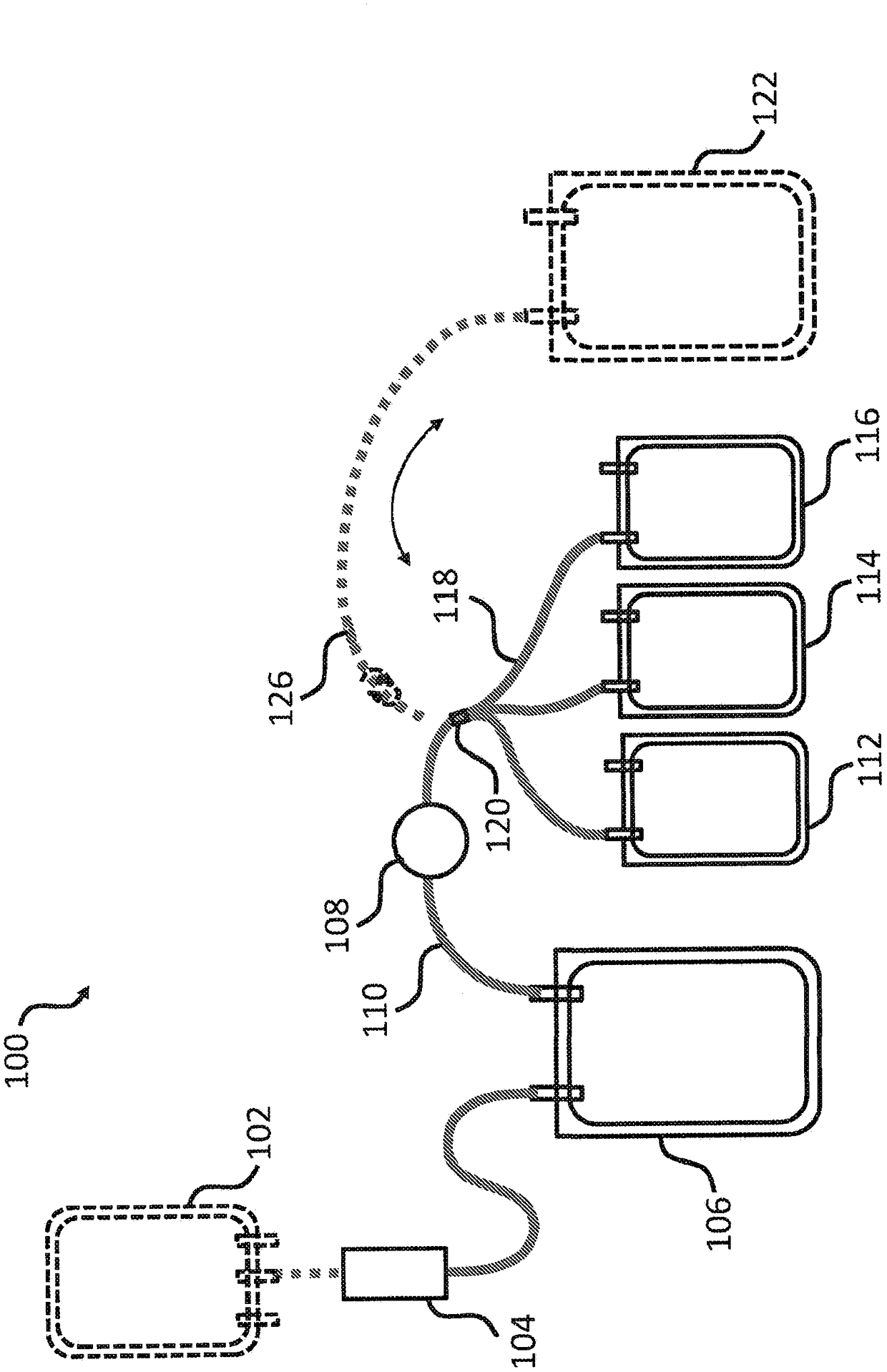
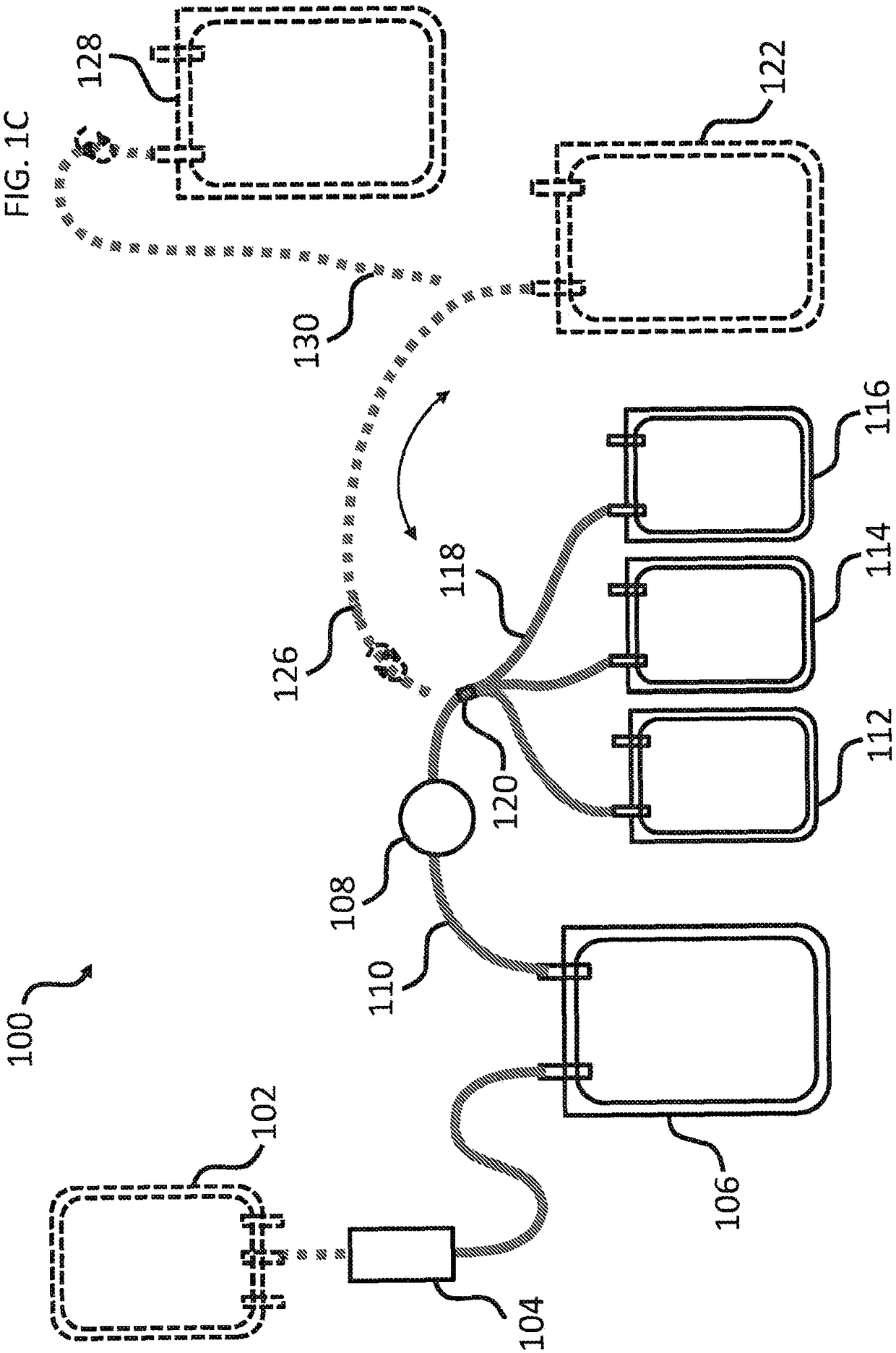
Cryoprecipitate compositions and methods of preparation thereof
Provided herein are compositions and kits including a pathogen-inactivated cryoprecipitate suitable for infusion into a subject at least 1 day after thawing. The methods are useful in the efficient preparation of cryoprecipitates with desirable characteristics, including pathogen-inactivated cryoprecipitates that are suitable for infusion into a subject at least 1 day after thawing.
Owner:CERUS CORP
A method for preparing egg yolk lecithin for injection by static adsorption of alumina to remove impurities and low-temperature precipitation for deoiling
The invention discloses a method for preparing egg yolk lecithin for injection through static adsorption of alumina to remove impurities and low-temperature precipitation and deoiling, and belongs to the technical field of egg yolk lecithin refining. The method includes the following steps: using egg yolk powder or egg yolk granules as raw materials, extracting with absolute ethanol, statically adsorbing aluminum oxide, dissolving with a mixed solution of n-hexane-acetone, precipitating lecithin at low temperature, and drying the precipitate to obtain the product. The obtained product was tested by thin-layer chromatography, and the residues of triglyceride, cholesterol and palmitic acid met the limit requirements of the Chinese Pharmacopoeia 2015 edition. The invention provides a brand-new process method for removing impurities and deoiling egg yolk lecithin, and the method has high yield and good effect of removing impurities and deoiling. Alumina static adsorption takes short working hours and is easy to operate, and can remove impurities such as LPE and PI smoothly; and the equipment is simple, the investment in fixed assets is small, the solvent is easy to recycle and reuse, and the discharge of three wastes is greatly reduced.
Owner:SHENYANG TIANFENG BIOLOGICAL PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com