Pre-purifying method for cryoprecipitate
A cryoprecipitation and pre-purification technology, which is applied in the direction of animal/human protein, VII factor, coagulation/fibrinolytic factor, etc., can solve the problem of ion exchange chromatography purification effect, gel service life realization, affecting the quality of loading liquid, purification Ineffective and other problems, achieve the effect of reducing instability and insecurity, reducing equipment investment, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
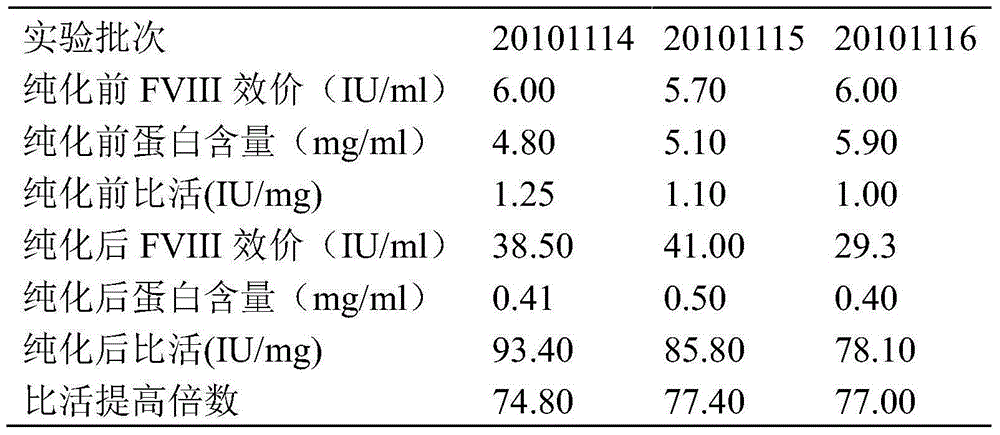
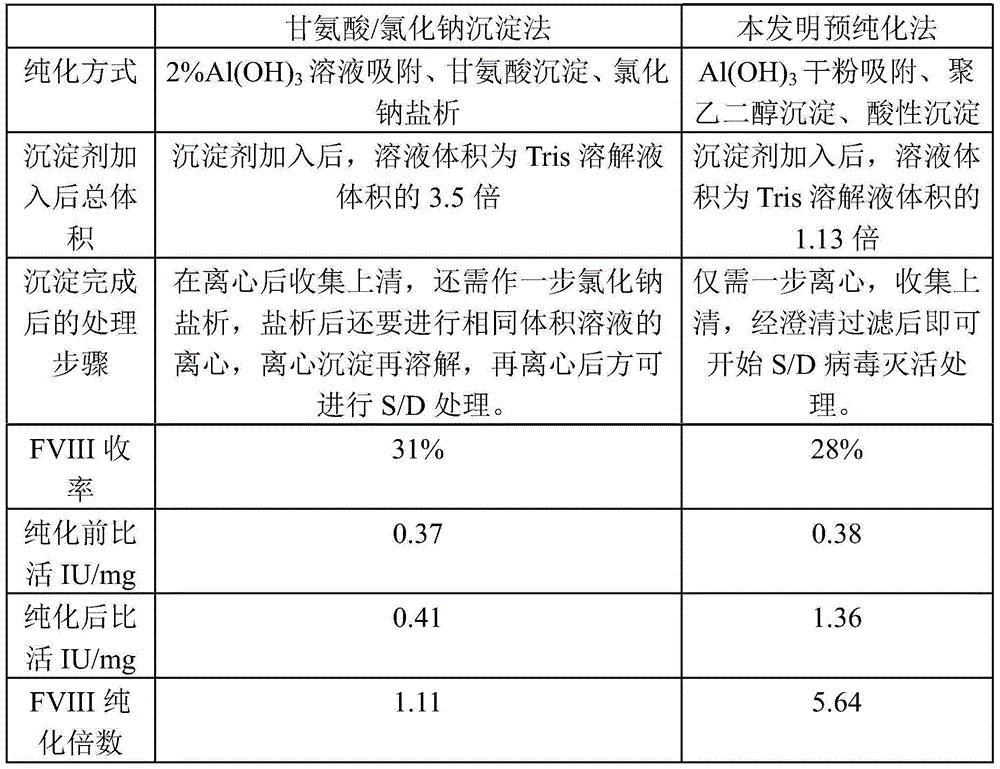
[0030] Embodiment 1 The prepurification method of cryoprecipitation of the present invention
[0031] (1) Take 25kg of cryoprecipitate
[0032] Cryoprecipitate refers to the plasma from healthy people stored below -20°C for not more than 1 year;
[0033] The cryoprecipitate should be a uniform milky white to yellowish viscous precipitate. After extraction, it should be stored under -60℃ and transported under -30℃.
[0034] (2) Add cryoprecipitate into 75L 20mmol / L pH6.8Tris solution under stirring at a ratio of 1:3, and the process temperature shall not be lower than 20℃;
[0035] (3) According to the cryoprecipitation of 5g aluminum hydroxide gel dry powder / kg, add 125g aluminum hydroxide gel dry powder to the cryoprecipitation solution. After adding, continue stirring for 15 minutes, and control the temperature to 20 °C;
[0036] (4) Add the polyethylene glycol mother solution to the above solution, so that the final concentration of polyethylene glycol 4000 is 3.0%. Afte...
Embodiment 2
[0039] Embodiment 2 The prepurification method of cryoprecipitation of the present invention
[0040] (1) Take 25kg of cryoprecipitate
[0041] Cryoprecipitate refers to the plasma from healthy people stored below -20°C for not more than 1 year;
[0042] The cryoprecipitate should be a uniform milky white to yellowish viscous precipitate. After extraction, it should be stored under -60℃ and transported under -30℃.
[0043] (2) Add cryoprecipitate into 75L 20mmol / L pH6.8Tris solution under stirring at a ratio of 1:3, and the process temperature shall not be lower than 20℃;
[0044] (3) According to the cryoprecipitation of 15g aluminum hydroxide gel dry powder / kg, add 375g aluminum hydroxide gel dry powder to the cryoprecipitation solution. After adding, continue to stir for 20 minutes, and control the temperature to 30°C;
[0045] (4) Add polyethylene glycol mother solution to the above solution, so that the final concentration of polyethylene glycol 4000 is 3.5%, stir even...
Embodiment 3
[0048] Embodiment 3 The prepurification method of cryoprecipitation of the present invention
[0049] (1) Take 25kg of cryoprecipitate
[0050] Cryoprecipitate refers to the plasma from healthy people stored below -20°C for not more than 1 year;
[0051] The cryoprecipitate should be a uniform milky white to yellowish viscous precipitate. After extraction, it should be stored under -60℃ and transported under -30℃.
[0052] (2) Add cryoprecipitate into 75L 20mmol / L pH6.8Tris solution under stirring at a ratio of 1:3, and the process temperature shall not be lower than 20℃;
[0053] (3) According to the cryoprecipitation of 15g aluminum hydroxide gel dry powder / kg, add 375g aluminum hydroxide gel dry powder to the cryoprecipitation solution. After adding, continue to stir for 20 minutes, and control the temperature to 35°C;
[0054] (4) Add the polyethylene glycol mother solution to the above solution, so that the final concentration of polyethylene glycol 4000 is 3.5%, and a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com