Cryoprecipitate compositions and methods of preparation thereof
A composition and sediment technology, applied in drug delivery, pharmaceutical formulations, packaging items, etc., can solve problems such as increased costs and product waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0155] Embodiment 1: the preparation of the cryoprecipitate of pathogen inactivation
[0156] Multiple liquid plasma units from O-negative blood units on the day of draw were pooled and separated to obtain multiple plasma units for processing, each having a final volume of 585 to 650 mL. Pathogen inactivation and preparation of cryoprecipitate using concentrated plasma units. The plasma was subjected to photochemical pathogen inactivation using a commercially available INTERCEPT Blood System for Plasma (Cerus Corporation). In the case of four pooled units for INTERCEPT processing, the pathogen-inactivated plasma collected in the storage bags of the 3 INTERCEPT processing modules was transferred to a single 1000 mL transfer bag as a bulk unit product. The other two pooled units undergoing INTERCEPT treatment were kept in 3 storage bags as a single "single unit" product of low volume. The pathogen-inactivated plasma units were then frozen at -30°C for cryoprecipitate preparati...
Embodiment 2
[0163] Embodiment 2: the preparation of the cryoprecipitate of pathogen inactivation
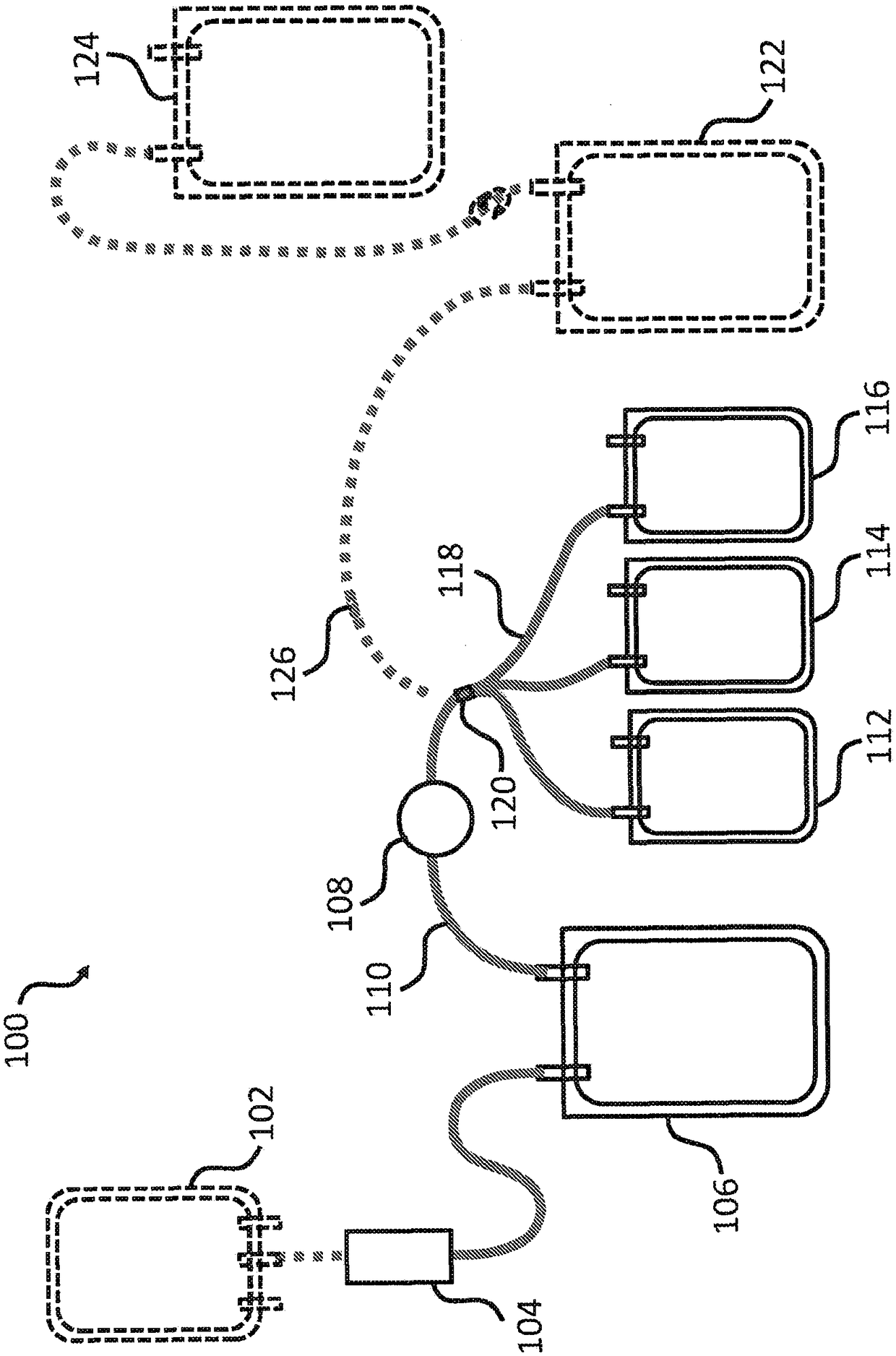
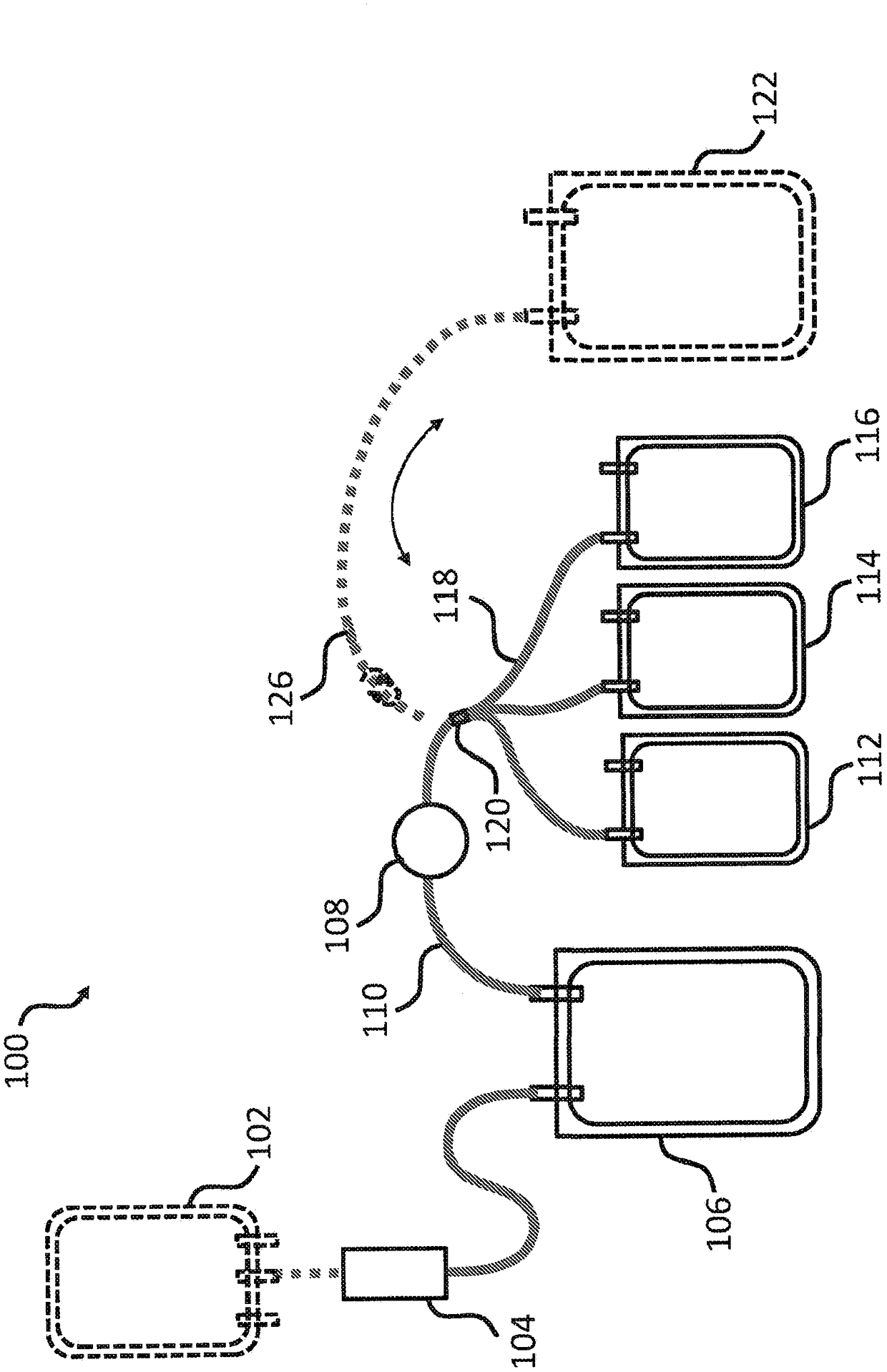
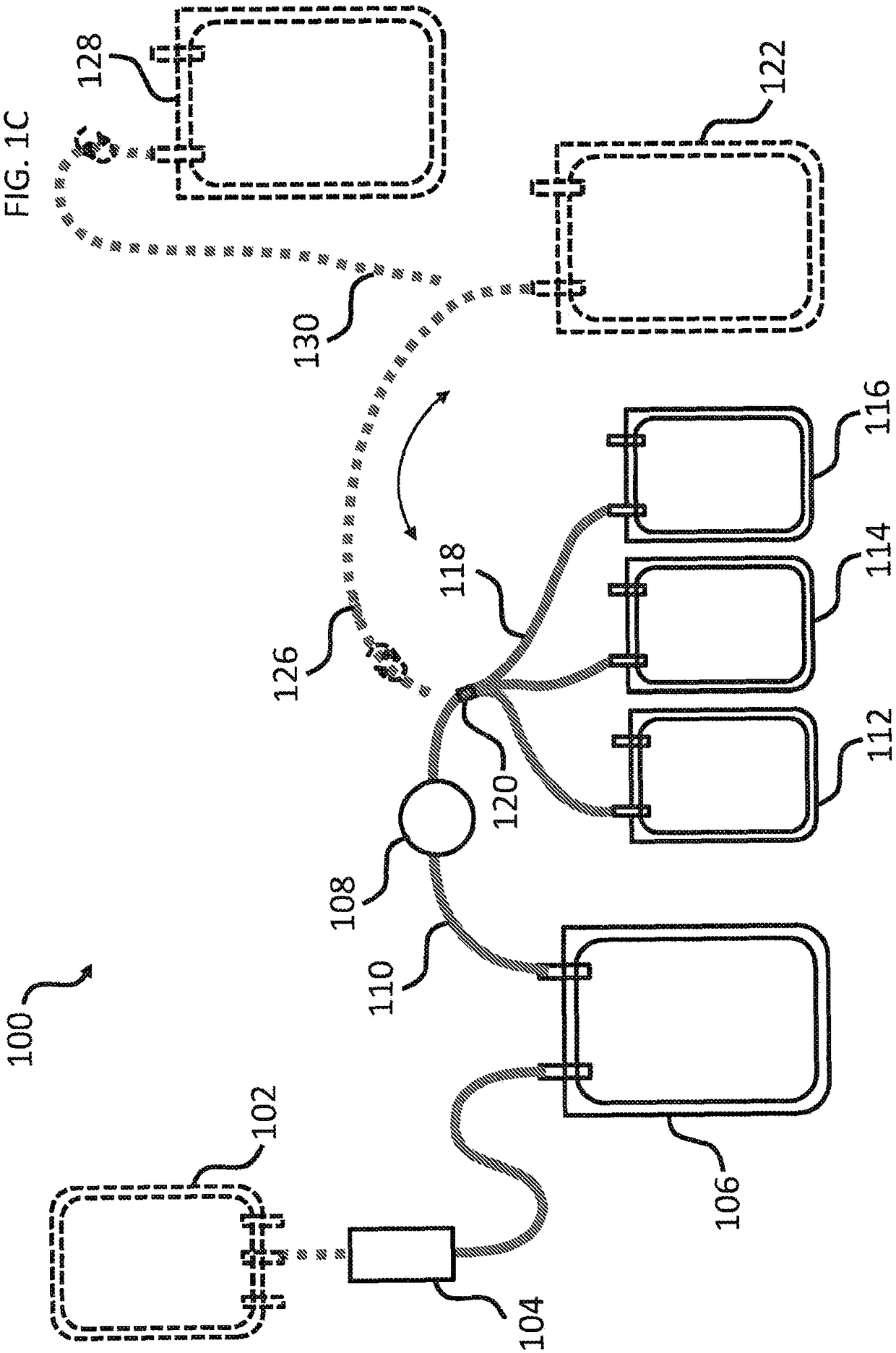
[0164] Multiple plasma units obtained from blood type A donors are pooled to obtain plasma preparations each having a volume (eg, large volume) of about 650 mL. Pathogen inactivation and preparation of cryoprecipitate using concentrated plasma units. Plasma was subjected to photochemical pathogen inactivation using a commercially available INTERCEPTBlood System for Plasma (Cerus Corporation), yielding three separate pathogen-inactivated plasma (PI plasma) from each pool. Combine three PI plasma units (3 containers, see e.g. Figure 1B 112, 114 and 116 in ), and frozen at -30°C for the preparation of cryoprecipitate.
[0165] For preparation of cryoprecipitate, the pooled units were thawed in a temperature-controlled 4°C water bath for a total thawing time of approximately 6 hours 15 minutes. Thawed units were centrifuged at 4200 rcf for 12 min at 4°C with slow deceleration. The cryopreci...
Embodiment 3
[0169] Example 3: Preparation of PI cryoprecipitate and cryoprecipitate-depleted plasma
[0170] Within 8 hours of draw, pathogen-inactivated (PI) cold blood samples were prepared from three large volume (647 ± 2 mL) feed pools of 2-3 units of ABO-matched whole blood-derived plasma in CPD anticoagulant. Precipitate and cryoprecipitate-depleted plasma supernatant.
[0171] The pooled plasma was subjected to photochemical pathogen inactivation using amtroxalin and UVA with a commercially available INTERCEPT Blood System for Plasma. Prior to INTERCEPT treatment, baseline samples were collected and subjected to pathogen inactivation according to the manufacturer's package insert. Pool three PI plasma units prepared from each INTERCEPT processing component (3 containers, see e.g. Figure 1B 112, 114 and 116 in ). After sampling, the pathogen-inactivated plasma preparations were snap frozen and stored at -30°C for cryoprecipitate preparation.
[0172] For cryoprecipitate prepara...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com