Method for preparing cryoprecipitate coagulation factor
A blood coagulation factor and cryoprecipitation technology, which is applied in blood diseases, medical formulas, extracellular fluid diseases, etc., can solve the problems of cryoprecipitate coagulation factors flowing into empty bags, failing to succeed, and taking a long time to achieve a high content of factor VIII , good clinical infusion effect and short preparation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0078] 1 Materials and methods
[0079] 1.1 Instruments, materials and reagents
[0080] 1.1.1 Instruments and materials:
[0081] Microwave oven (Panasonic-NN-CS957S);
[0082] DLM12L ultra-large capacity refrigerated centrifuge (Changsha Xiangzhi);
[0083] Water-bath low-temperature melter (Dakewei Biotechnology Co., Ltd.);
[0084] Refrigerator (Haier Company);
[0085] High-efficiency heat sealing machine (Shenzhen Dakowei Medical Technology Co., Ltd.);
[0086] Electronic scale (Shanghai Jingtian Electronic Instrument Co., Ltd.);
[0087] BE-THROMBOTIMER4 blood coagulation instrument (German BE company);
[0088] The 400ml five-pack preservation solution is CPDA-1 anticoagulant (Shandong Weigao).
[0089] 1.1.2 Reagents:
[0090] IBS buffer, lot number 036N-D064A; FactorVIII, lot number 11237812; APTT-EA, lot number 109130410; CaCl 2 , batch number 11108071; fibrinogen assay kit, batch number 11129124; a full set of reagents are provided by German Metron.
[00...
Embodiment 1
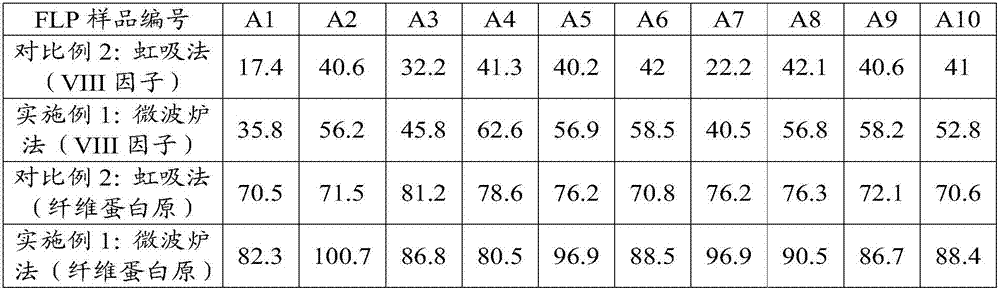
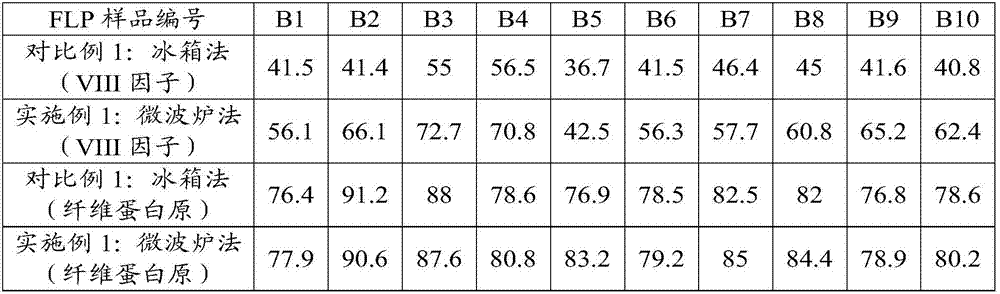
[0104] (3) Embodiment 1: microwave oven melting centrifugation (the present invention):
[0105] Weigh 10 copies of FFP in groups of 5, turn on the microwave oven, spread 5 bags of fresh frozen plasma in the microwave box, select the temperature control function, set the temperature: 5°C, select the steam thawing program, enter Weight, start thawing. After thawing, the ice-slag-like mixture was placed in a refrigerated centrifuge at a temperature of 2°C±2°C and a centrifugal force of 3800g, and centrifuged for 10min. Separate the upper layer of plasma, and the rest of 20-30ml is the cryoprecipitate.
[0106] 1.2.3. Detection method of factor VIII and fibrinogen:
[0107] Strictly follow the operating instructions of the hemagglutination analyzer produced by BE Company and the instructions of German Metron reagents.
[0108] 1.2.4 Capacity:
[0109] Weigh with an electronic scale.
[0110] 1.2.5 Quality standard:
[0111] Cryoprecipitate from 200ml whole blood: Factor VII...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com