Production method of fibrinogen
A fibrinogen and production method technology, applied in the field of biopharmaceuticals, can solve problems such as the influence of reconstitution time, expensive medicines, and reduced coagulation activity, and achieve good appearance and stability, good coagulation activity, and short reconstitution time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
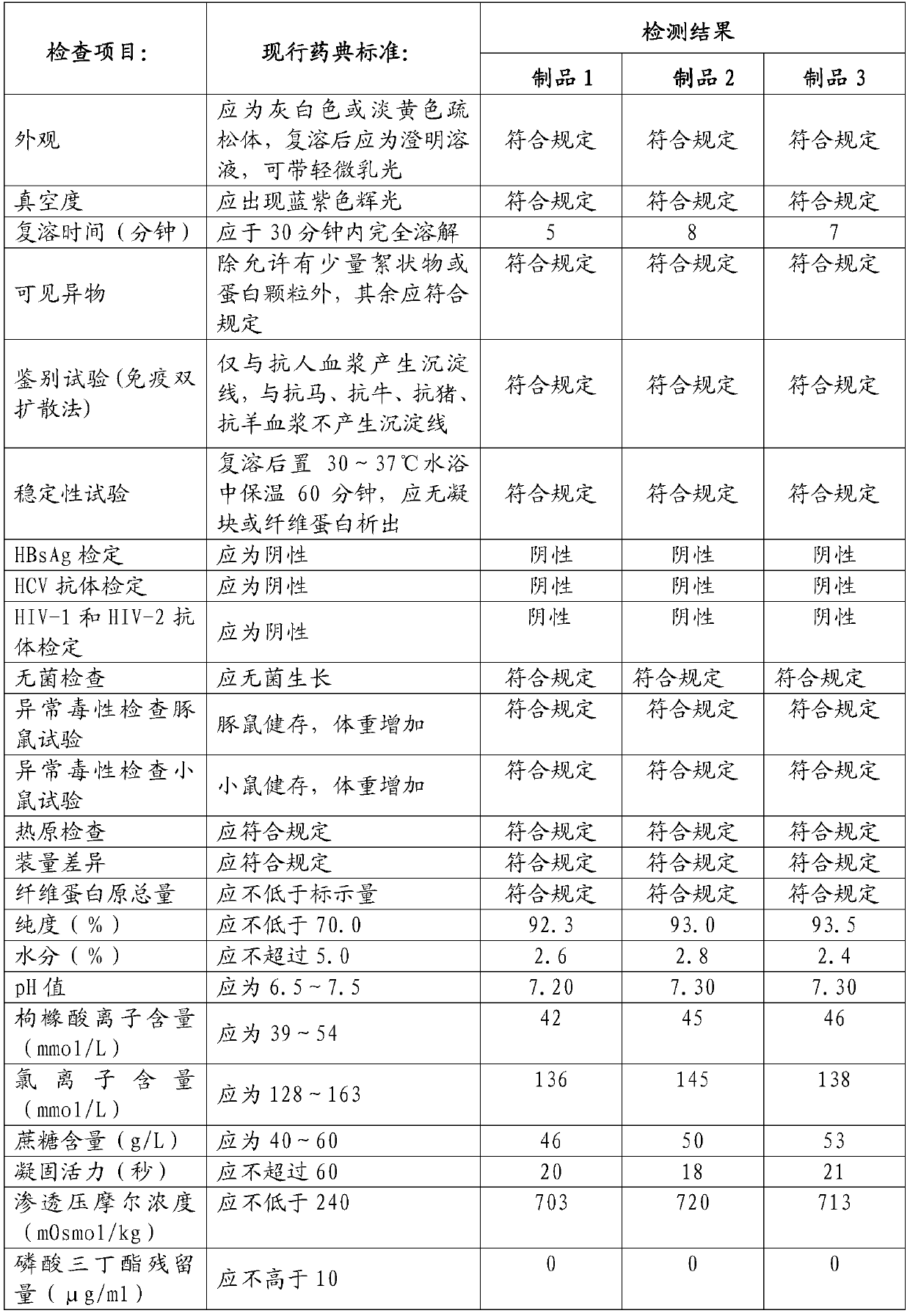
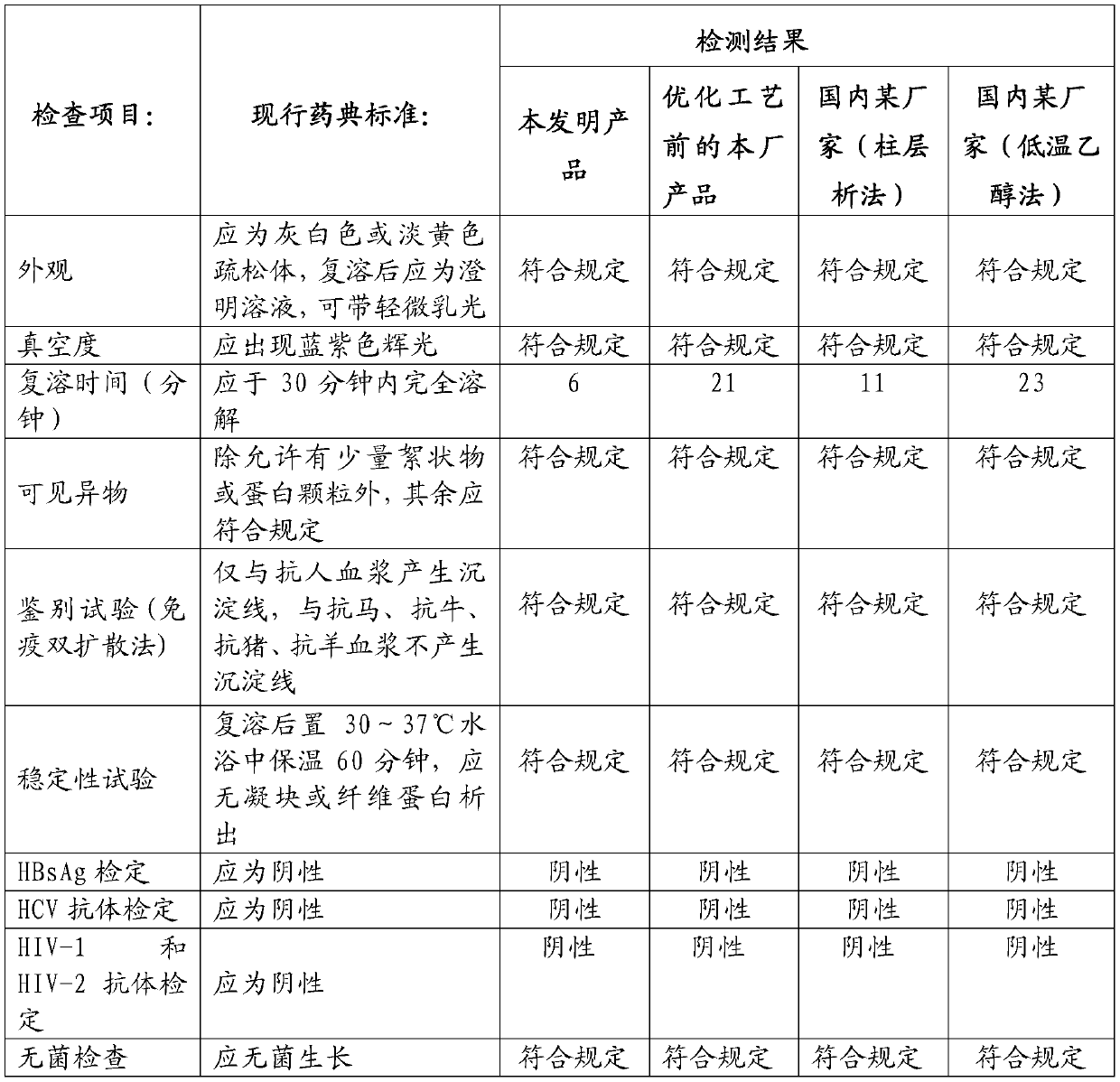
Embodiment 1
[0107] (1) Plasma collection and quick-freezing: 3.48 tons of fresh human plasma collected was quick-frozen within 30 minutes by flat-plate direct-cooling quick-freezing technology and stored in a -30°C freezer. This batch of plasma passed the virus test and met the quarantine period stipulated by the state.
[0108](2) Plasma melting and cryoprecipitation: transfer the batch of qualified plasma from the -30°C freezer to the pre-thawing room at 0-4°C for 1 hour, and then spray it with water for injection below 10°C. Alcohol spray disinfection and water for injection rinse alcohol and dry. Break the bag of the raw material plasma and transfer it to the slurry melting tank, circulate the water for injection with an interlayer temperature of 30°C to melt the plasma, and perform continuous separation and centrifugation at a speed of 5,000-5,600 rpm. The liquid intake of the centrifuge is 900 L / min. kg and the supernatant, and stored in a -30°C freezer.
[0109] (3) Preparation o...
Embodiment 2
[0122] (1) Plasma collection and quick-freezing: 3.51 tons of fresh human plasma collected was quick-frozen within 30 minutes by flat-plate direct-cooling quick-freezing technology and stored in a -30°C freezer. This batch of plasma passed the virus test and met the quarantine period stipulated by the state.
[0123] (2) Plasma melting and cryoprecipitation: transfer the batch of qualified plasma from the -30°C cold storage to the pre-thawing room at 0-4°C for 1.5 hours, and then spray it with water for injection below 10°C. Alcohol spray disinfection and water for injection rinse alcohol and dry. Break the bag of the raw material plasma and transfer it to the melting tank, and circulate the water for injection with an interlayer temperature of 30°C to melt the plasma, and perform continuous separation and centrifugation at a speed of 5000-5600 rpm. kg and the supernatant, and stored in a -30°C freezer.
[0124] (3) Preparation of Component I Precipitation: Transfer the supe...
Embodiment 3
[0137] (1) Plasma collection and quick-freezing: 3.45 tons of fresh human plasma collected was quick-frozen within 30 minutes by flat-plate direct-cooling quick-freezing technology and stored in a -30°C freezer. This batch of plasma passed the virus test and met the quarantine period stipulated by the state.
[0138] (2) Plasma melting and cryoprecipitation: transfer the batch of qualified plasma from the -30°C freezer to the pre-thawing room at 0-4°C for 2 hours, and then spray it with water for injection below 10°C. Alcohol spray disinfection and water for injection rinse alcohol and dry. Break the bag of the raw material plasma and transfer it to the slurry tank, and circulate the water for injection with an interlayer temperature of 30°C to melt the plasma, and perform continuous separation and centrifugation at a speed of 5000-5600 rpm. kg and the supernatant, and stored in a -30°C freezer.
[0139] (3) Preparation of Component I Precipitation: Transfer the supernatant ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com