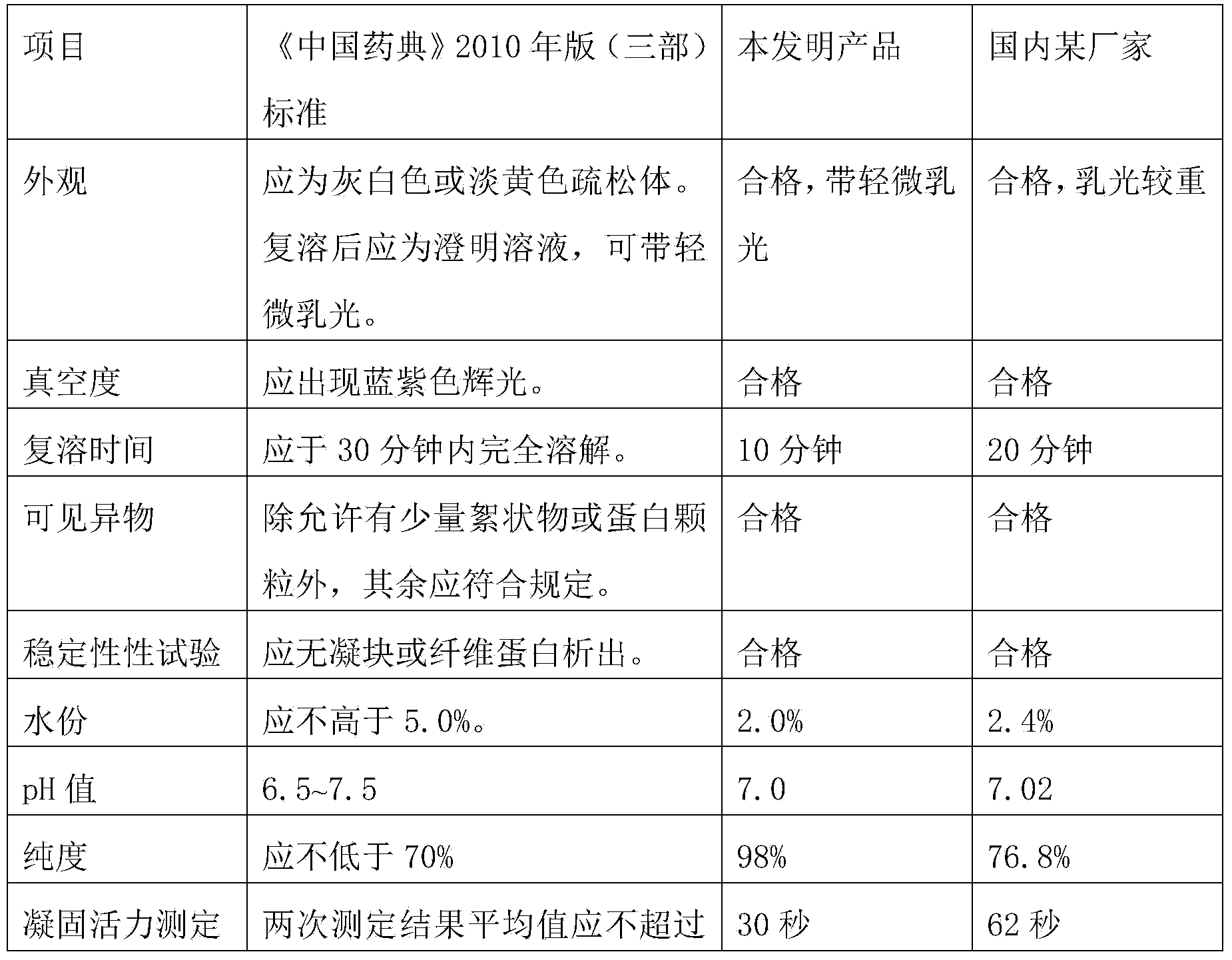
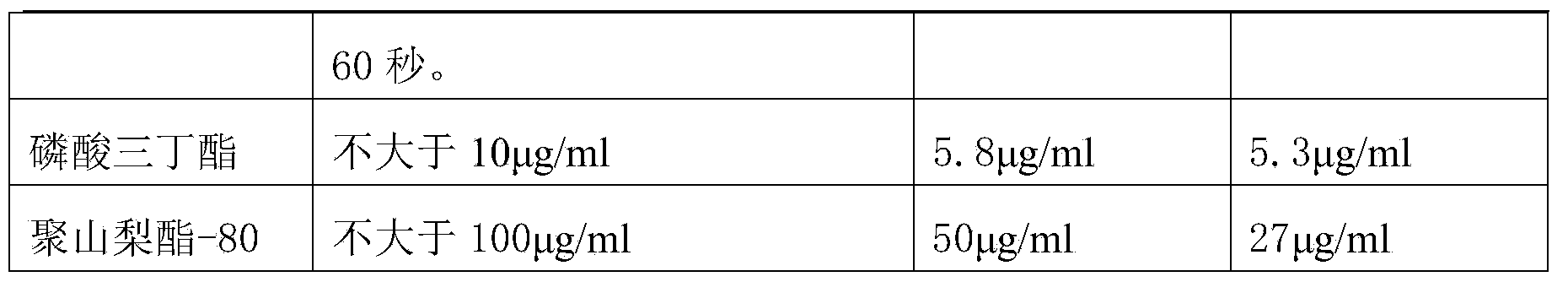
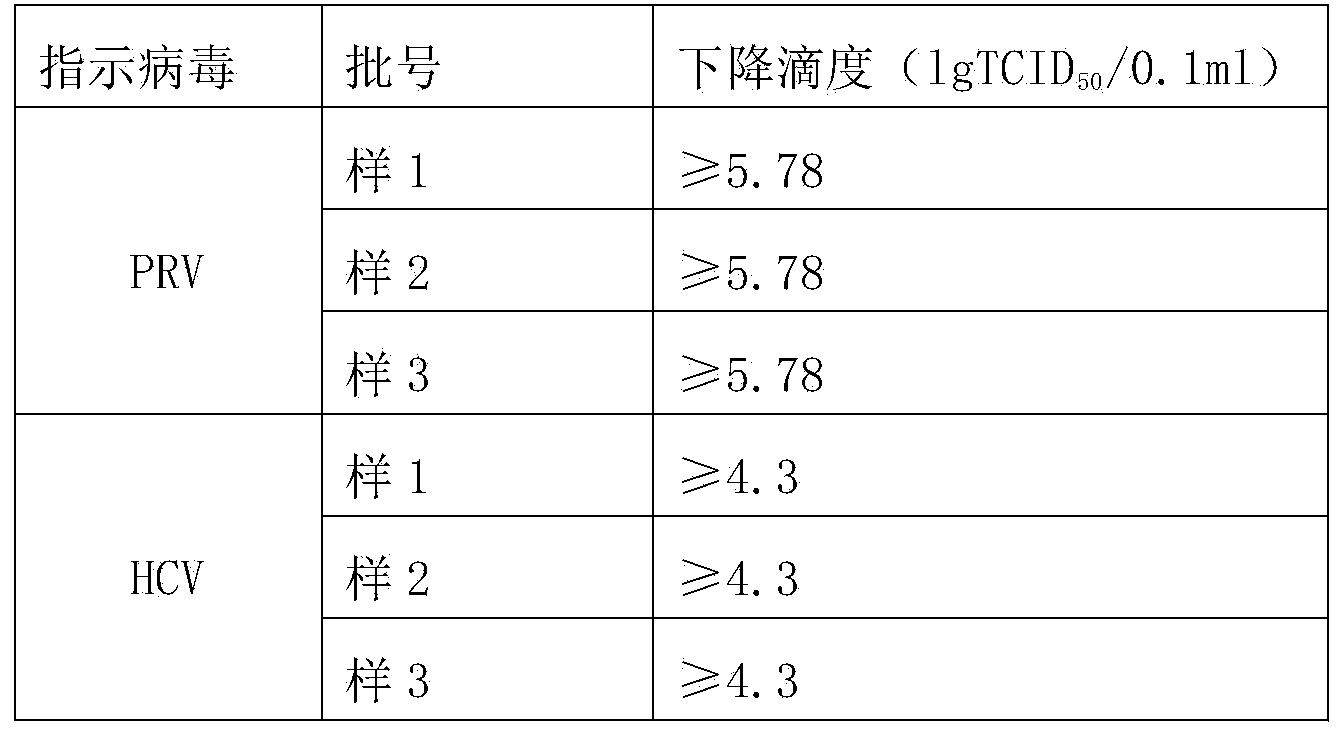
Solubilization technology for producing human fibrinogen
A human fibrinogen, process technology, applied in the direction of fibrinogen, animal/human protein, coagulation/fibrinolytic factor, etc., can solve the problem of low solubility of human fibrinogen precipitation, prone to adverse reactions, and high content of impurities in the product and other problems, to achieve the effect of stable temperature change, rapid dissolution, and increased solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Plasma selection:
[0030] According to the production plan, the frozen plasma was weighed in the -20°C freezer and shipped out. Screen the frozen plasma out of the warehouse to remove damaged plasma. Qualified plasma is washed and placed in a clean stainless steel barrel, and the turnover box is built to 4-5 layers, and placed in a 2-15°C freezer overnight.
[0031] 2. Plasma collection: first wash with water for injection below 37°C to make the plasma reach the state of shelling; then sterilize with 75-80% ethanol, and replace with new alcohol after every 4 to 5 boxes of plasma are sterilized. Then remove the plasma bag to the washing table, and fully rinse the surface of the plasma bag with water for injection below 37°C. Break the bag, collect the plasma in a special plasma collection tank with an interlayer, heat it with circulating water at 35°C, transfer the melted plasma to a special centrifuge tank in time, and control the temperature of the plasma at 4°C,...
Embodiment 2
[0041] 1. Plasma selection:
[0042] According to the production plan, the frozen plasma was weighed from the -20°C freezer and shipped out. Screen the frozen plasma out of the warehouse, and pick out the damaged plasma. The damaged plasma should be picked out in time, washed and placed in a clean stainless steel bucket. Damaged plasma cannot produce cryoprecipitate; build the turnover box to 4-5 layers, and put the plasma in a 2-8 degree freezer overnight.
[0043] 2. Plasma collection: first wash with water for injection below 37°C to make the plasma reach the shell state; then sterilize with 75-80% ethanol. After every 4 to 5 boxes of plasma are sterilized, replace with new alcohol, and then remove the plasma bag to the washing table. Then fully rinse the surface of the plasma bag with water for injection below 37°C. Break the bag, collect the plasma in a special plasma collection tank with an interlayer, heat it with circulating water at 30°C, transfer the melted plasma...
Embodiment 3
[0052] 1. Plasma selection:
[0053] According to the production plan, the frozen plasma was weighed from the -20°C freezer and shipped out. Screen the frozen plasma out of the warehouse, and pick out the damaged plasma. The damaged plasma should be picked out in time, washed and placed in a clean stainless steel bucket. Damaged plasma cannot produce cryoprecipitate; build the turnover box to 4-5 layers, and put the plasma in a 2-8 degree freezer overnight.
[0054] 2. Plasma collection: first wash with water for injection below 37°C to make the plasma reach the shell state; then sterilize with 75-80% ethanol. After every 4 to 5 boxes of plasma are sterilized, replace with new alcohol, and then remove the plasma bag to the washing table. Then fully rinse the surface of the plasma bag with water for injection below 37°C. Break the bag, collect the plasma in a special plasma collection tank with an interlayer, heat it with circulating water at 25°C, transfer the melted plasma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com