Production process of blood-derived human blood coagulation factor VIII/von willebrand factor compound
A technology of human coagulation factor and hemophilia factor, which is applied in the direction of coagulation/fibrinolytic factor, VII factor, animal/human protein, etc., can solve problems such as complex process, maintain binding ability, prevent titer attenuation, prevent activated effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
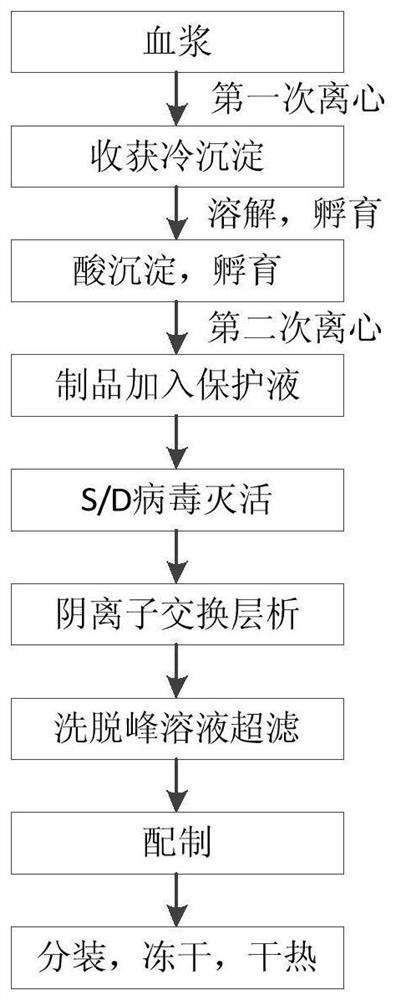
[0060] Such as figure 1 As shown, a FⅧ / VWF complex production process includes the following steps:
[0061] (1) Preparation of cryoprecipitate from plasma, dissolution of cryoprecipitate, and removal of crude impurities
[0062] Plasma cryoprecipitate was prepared by centrifugation with a centrifugal force of 5500g. 50kg of cryoprecipitate was stirred and dissolved with 100kg of water for injection containing 1U / ml heparin at 35°C. Use 0.1mol / L acetic acid to adjust the pH value of the solution to 6.90, the conductivity to 3.8mS / cm, stir at 25°C to keep the insoluble matter in the product in a suspended state, and incubate for 1 hour. After the incubation, adjust the temperature of the product to 15°C. 0.1mol / L acetic acid to adjust the pH value of the product to 6.50, the pH adjustment process takes 1 hour, stir to keep the acid precipitate in a suspended state, and incubate for 3 hours. The acid precipitate is separated by centrifugation with a centrifugal force of 5500g...
Embodiment 2
[0087] Such as figure 1 As shown, a FⅧ / VWF complex production process includes the following steps:
[0088] (1) Preparation of cryoprecipitate from plasma, dissolution of cryoprecipitate, and removal of crude impurities
[0089]Plasma cryoprecipitate was prepared by centrifugation at a centrifugal force of 1000g. 50kg of cryoprecipitate was stirred and dissolved with 150kg of water for injection containing 1U / ml heparin at 35°C. Use 0.1mol / L acetic acid to adjust the pH value of the solution to 6.60, the conductivity to 3.2mS / cm, stir at 25°C to keep the insoluble matter in the product in a suspended state, and incubate for 1 hour. After the incubation, adjust the temperature of the product to 15°C. 0.1mol / L acetic acid was used to adjust the pH value of the product to 6.30. The pH adjustment process took 3 hours, stirred to keep the acid precipitate in a suspended state, and incubated for 3 hours. The acid precipitate was separated by centrifugation, centrifugal force 100...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com