Production method of recombinant human blood coagulation factor XIIa
A technology of human blood coagulation factor and production method, which is applied in the production field of recombinant human blood coagulation factor Ⅻa, can solve the problems of inability to carry out recombinant expression and purification, expensive raw materials, low yield, etc., achieve simplified quality control, and improve product quality , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
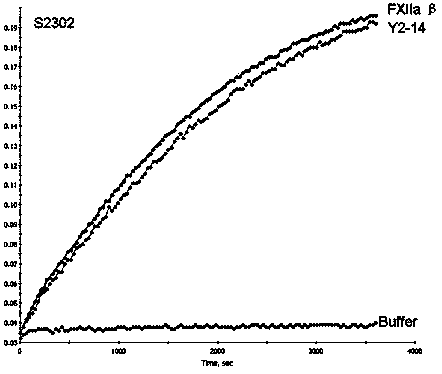
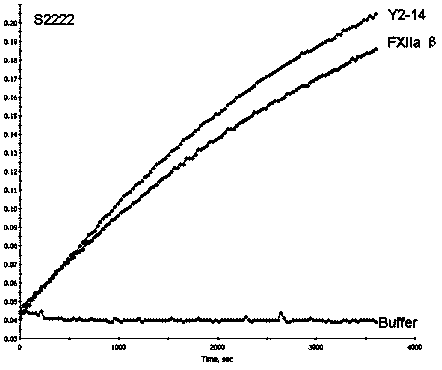
Image
Examples
Embodiment 1
[0024] The DNA (Sequence Listing 1) encoding coagulation factor XIIa (354-615) obtained by gene synthesis was cloned into the pPICZαB vector to construct the pPICZαB-coagulation factor XIIa vector, which was transformed into Escherichia coli DH5α. The recombinant plasmid pPICZαB-coagulation factor Ⅻa was extracted to a concentration of 736.28 μg / ml. Take 20 μg of the recombinant plasmid pPICZαB-coagulation factor Ⅻa for linearization by Sac Ⅰ single enzyme digestion, and dissolve it in TEBuffer after recovery, with a final volume of 30 μl. Inoculate X33 overnight culture into 10ml YPD liquid medium, 30°C, 230rpm, OD600=1.366 after about 16h. Centrifuge at 4°C, wash with ice sterile water and ice 1M sorbitol successively to resuspend the precipitate. Bring the final volume to 1.5ml. Take 60 μl of the above bacterial solution, mix it with the linearized DNA, and transfer it to an ice-cooled 0.2 cm electroshock cuvette. Ice bath for 5min. Select the yeast preset program for el...
Embodiment 2
[0026]The DNA (sequence list 3) encoding blood coagulation factor Ⅻa (354-615, C486A, that is, the 486th amino acid of blood coagulation factor Ⅻa mutated from cysteine to alanine) obtained by gene synthesis was cloned into the pPICZαB vector, and constructed pPICZαB-coagulation factor Ⅻa vector, transformed into Escherichia coli DH5α. The recombinant plasmid pPICZαB-coagulation factor Ⅻa was extracted to a concentration of 752.11 μg / ml. Take 20 μg of the recombinant plasmid pPICZαB-coagulation factor Ⅻa for Sac Ⅰ single enzyme digestion and linearization, and dissolve it in TE Buffer after recovery, with a final volume of 30 μl. Inoculate X33 overnight culture into 10ml YPD liquid medium, 30°C, 230rpm, OD600=1.366 after about 16h. Centrifuge at 4°C, wash with ice sterile water and ice 1M sorbitol successively to resuspend the precipitate. Bring the final volume to 1.5ml. Take 60 μl of the above bacterial solution, mix it with the linearized DNA, and transfer it to an ice-...
Embodiment 3
[0028] The coagulation factor Ⅻa (354-615, N433A, C486A) encoded by gene synthesis; that is, the 433rd amino acid of coagulation factor Ⅻa is mutated from asparagine to alanine; the 486th amino acid is mutated from cysteine to alanine Acid) DNA (sequence table 5) was cloned into the pPICZαB vector to construct the pPICZαB-coagulation factor Ⅻa vector, which was transformed into Escherichia coli DH5α. The recombinant plasmid pPICZαB-coagulation factor Ⅻa was extracted to a concentration of 717.52 μg / ml. Take 20 μg of the recombinant plasmid pPICZαB-coagulation factor Ⅻa for SacⅠ single enzyme digestion and linearization, and dissolve it in TE Buffer after recovery, with a final volume of 30 μl. Inoculate X33 overnight culture into 10ml YPD liquid medium, 30°C, 230rpm, OD600=1.366 after about 16h. Centrifuge at 4°C, wash with ice sterile water and ice 1M sorbitol successively to resuspend the precipitate. Bring the final volume to 1.5ml. Take 60 μl of the above bacterial sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com