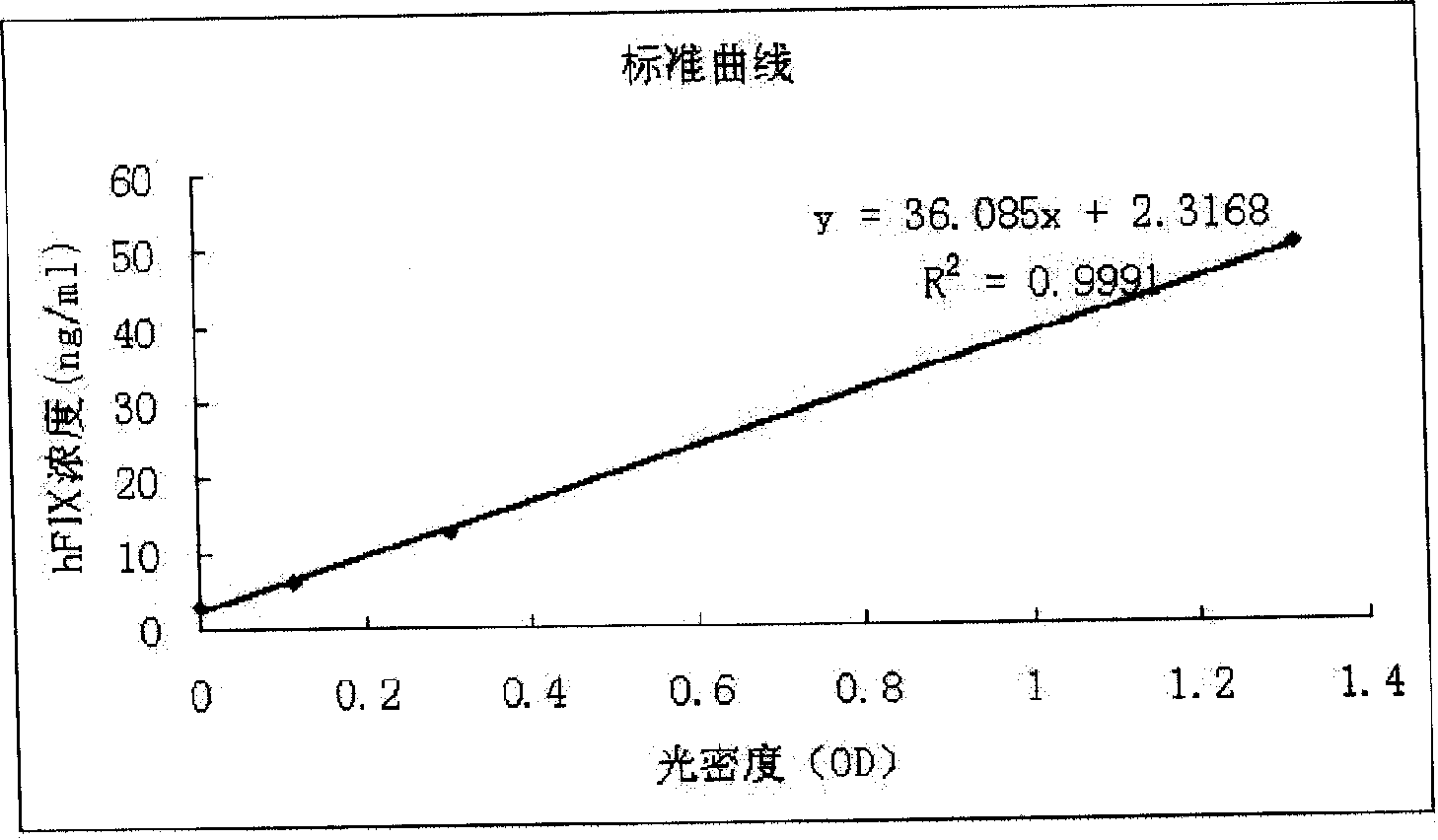
Non-blood serum preparation of recombinant human blood coagulation factor IX
A serum-free, human technology, applied in the field of preparation of recombinant hFIX, achieves good application effect, stable treatment, and improved production yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
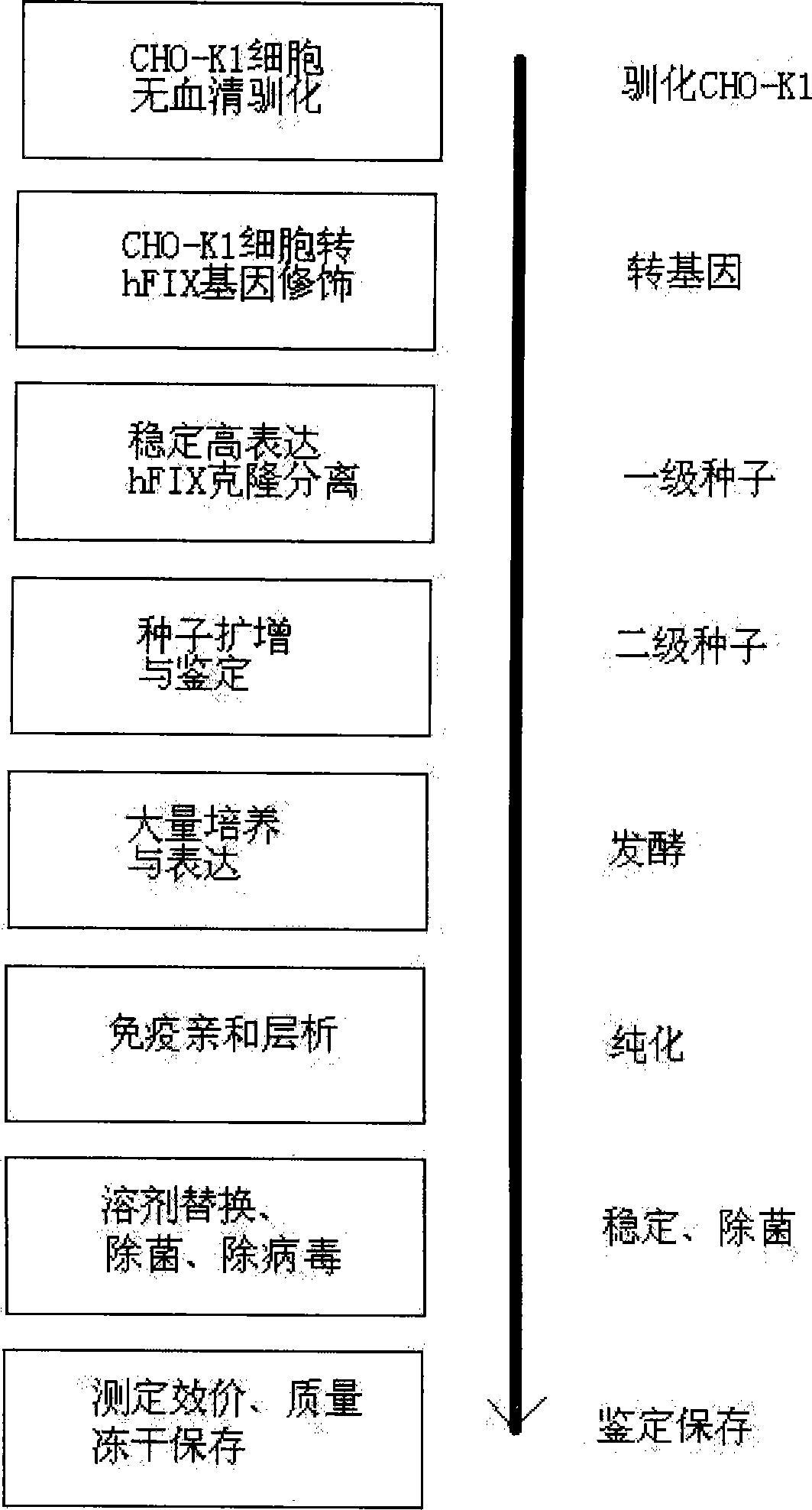
[0031] 1. Serum-free acclimatization of CHO-K1
[0032] CHO-K1 cells (Chinese hamster ovary cell K1 strain) were purchased from the American Type Culture Collection (CCL-61 TM , CHO-K1). Frozen cells were thawed in a water bath at 42°C, and the cells were washed with serum-free, and directly washed with 5ml of serum-free medium (serum-free medium was purchased from Gibco, CHO-S-SFM) at 37°C, 5% CO 2Cultivate under the same conditions, change the same medium every other day, digest with recombinant trypsin (Promga) when there are obvious clonal colonies, dilute the cells to 103 / ml, and inoculate them into 96-well cell culture plates respectively. Screen the cells with fast growth, large cell volume and no toxic particles as CHO-K1 serum-free suitable cells, and expand and save.
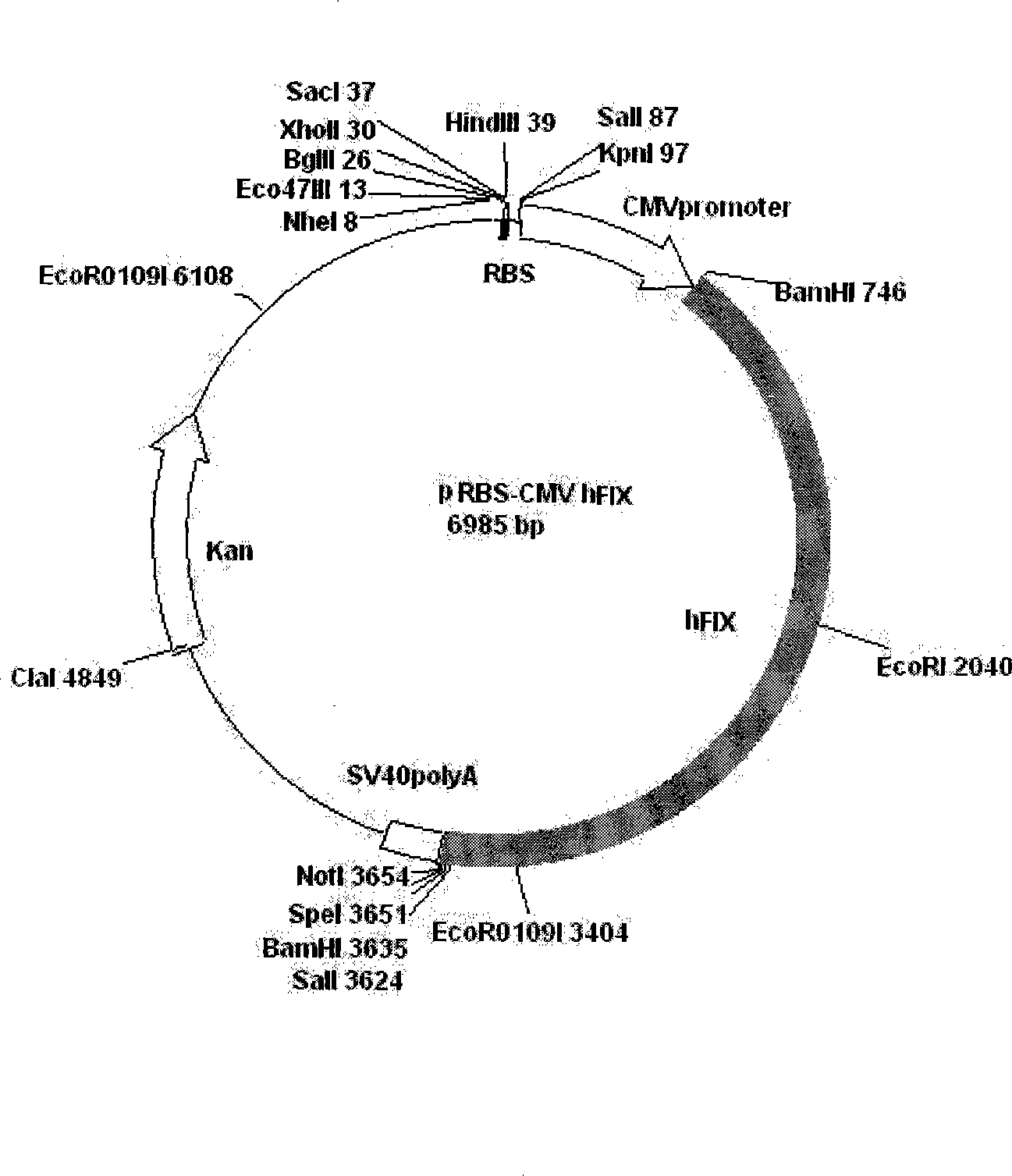
[0033] 2. Gene modification of CHO-K1 transfected with hFIX
[0034] The plasmid (pRBS-CMVhFIX) expressing hFIX was constructed by our laboratory, and its construction method is a conventional vecto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com