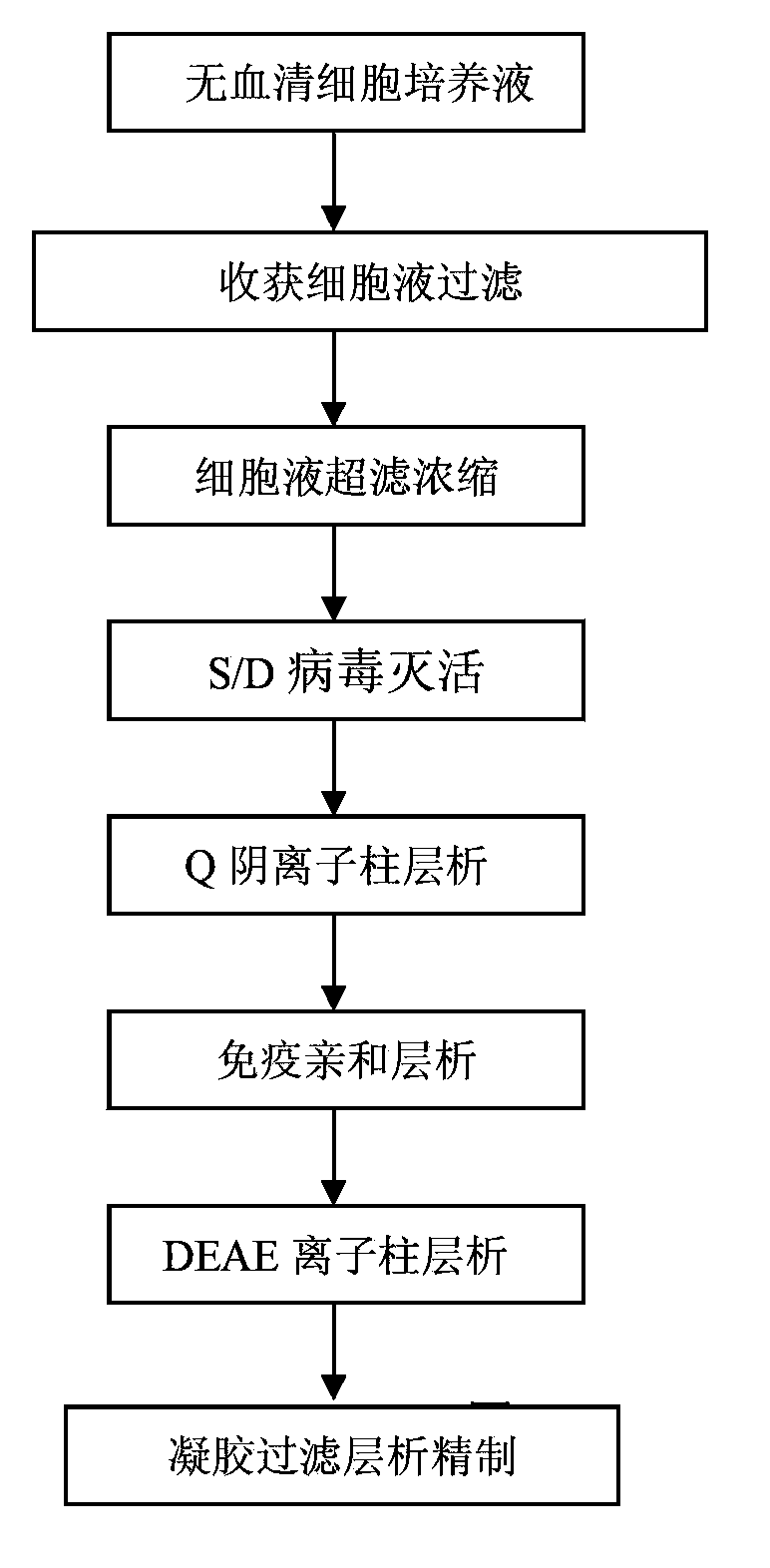
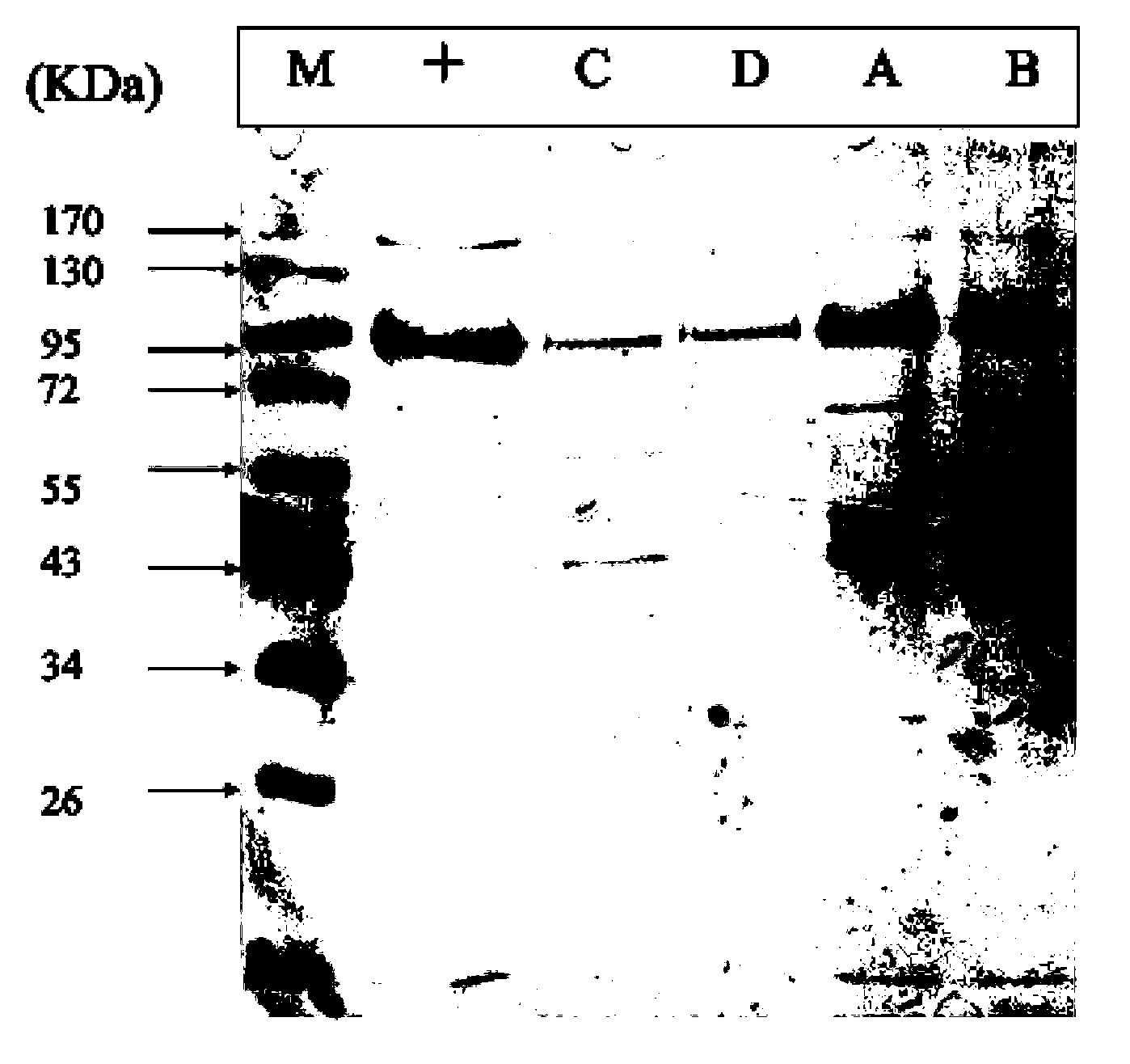
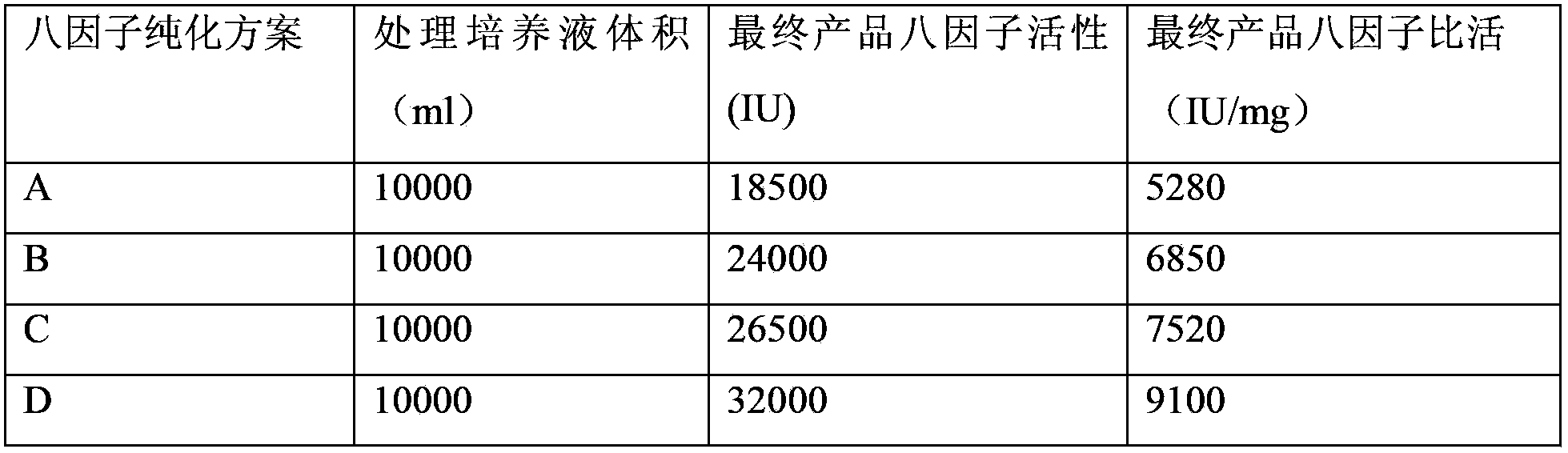
Method for separating and purifying recombinant human coagulation factor VIII from cell culture fluid
A cell culture, separation and purification technology, applied in the field of separation and purification of recombinant human coagulation factor eight, can solve the problems of easy degradation, poor stability and inactivation of eight factors, and achieve the goal of increasing specific activity, increasing stability and improving work efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1. Purpose of the test:
[0042] The effect of divalent metal ions, especially calcium ions and zinc ions on the stability of the eight factors during the purification process of the eight factors was investigated.
[0043] The experimental design investigated the stability of the eight factors under the conditions of adding calcium ions alone, adding zinc ions alone and adding calcium ions and zinc ions at the purification stage of the eight factors.
[0044] 2. Main material:
[0045] Recombinant human blood coagulation factor eight cell culture medium: Recombinant human blood coagulation factor eight CHO engineered cells (prepared according to the prior art) were obtained by culturing in SFMII302 medium (provided by SIGMA).
[0046] Q Sepharose FF ion-exchange chromatography column: GE Company of the United States
[0047] Eight-factor affinity (V8 SELECT) chromatography column: GE Company
[0048] DEAE FF ion exchange column: American GE company
[0049] Sephacr...
Embodiment 2
[0074] 1. Purpose of the test: To investigate the effects of different concentrations of calcium and zinc ions on the stability of recombinant human coagulation factor 8
[0075] 2. Main material: same as embodiment 1
[0076] 3. Experimental steps:
[0077] The purification scheme is the same as Scheme A of Example 1, except that the concentrations of calcium chloride and zinc acetate added to all buffers are different, as shown in Table 3.
[0078] The impact of different calcium chloride and zinc acetate concentrations on the stability of recombinant factor eight in the chromatography buffer of table 3
[0079] Program
Calcium chloride concentration in buffer (mM)
Zinc acetate concentration in buffer (mM)
E
1
1
F
10
10
G
50
50
H
100
100
[0080] 4. Result inspection: same as embodiment 1
[0081] 5. The results are shown in Table 4:
[0082] The impact result of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com