Preparation method of human coagulation factor VIII
A technology of human blood coagulation factor and plasma, which is applied in the field of preparation of human blood coagulation factor VIII, can solve problems such as production process optimization, and achieve the effect of saving resources and increasing efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
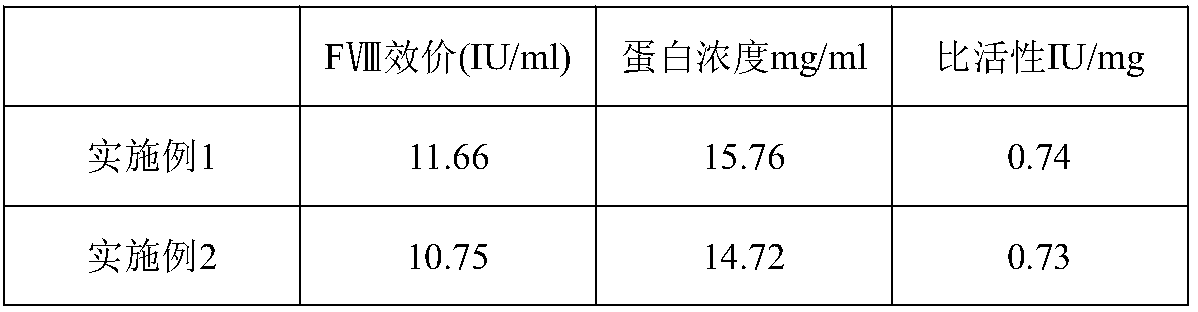
[0034] A preparation method for human blood coagulation factor VIII, comprising the steps of:
[0035] 1) Preparation of cryoprecipitate: prepare plasma cryoprecipitate;
[0036]2) Dissolving: cryoprecipitate is dissolved to obtain a solution;
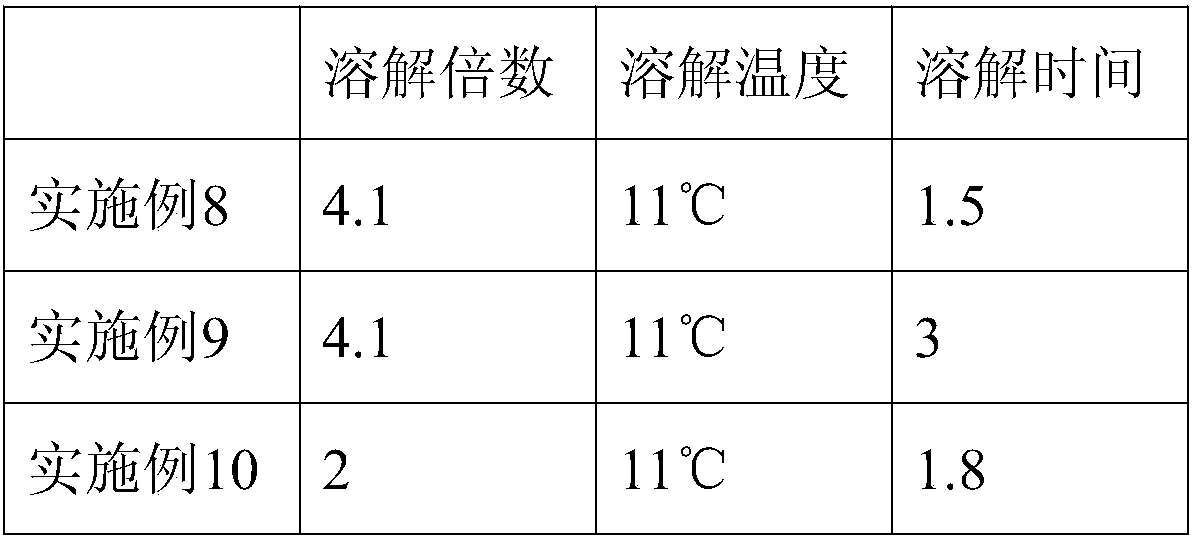
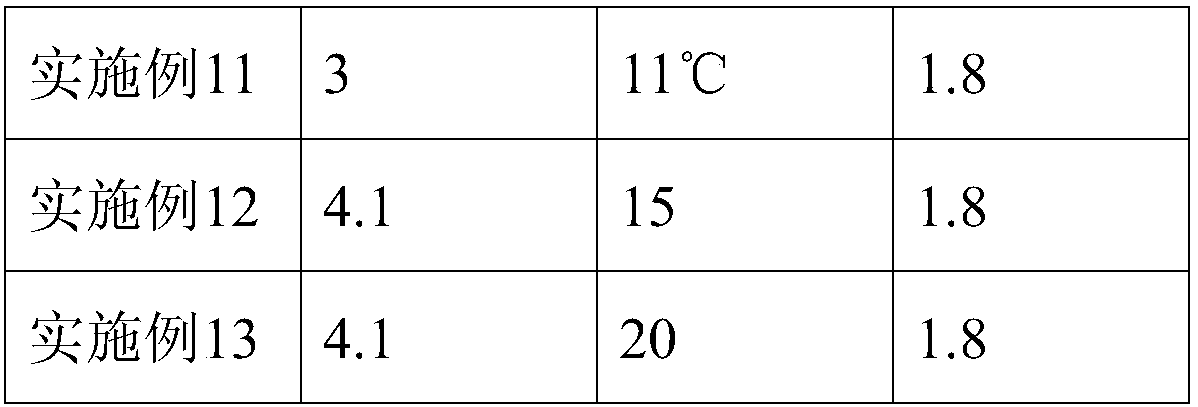
[0037] 3) Acid precipitation: adjust the pH of the solution with acetic acid solution, and lower the temperature to 14°C, collect the supernatant by centrifugation after acid precipitation, the liquid temperature is 15°C, and the liquid output speed of the centrifuge is ≤5000ml / min;
[0038] 4) DEAE Sephadex A-50 gel adsorption: Add DEAE Sephadex A-50 gel to the supernatant obtained in step 3) for adsorption, the amount of A-50 gel is 0.0025 kg per kilogram of cryoprecipitate dry gel, A -50 dry glue soaked in swelling solution for more than 3 hours before use, and balanced with the balance solution for 3 times, each time not less than 10 minutes, the adsorption pH is 6.9, and the adsorption time is 60 minutes. The output speed of eac...
Embodiment 2
[0056] A preparation method for human blood coagulation factor VIII, comprising the steps of:
[0057] 1) Preparation of cryoprecipitate: prepare plasma cryoprecipitate;
[0058] 2) Dissolving: cryoprecipitate is dissolved to obtain a solution;
[0059] 3) Acid precipitation: adjust the pH of the solution with acetic acid solution, and lower the temperature to 12°C. After the acid precipitation, centrifuge to collect the supernatant, the liquid temperature is 10°C, and the liquid output speed of the centrifuge is ≤5000ml / min;
[0060] 4) DEAE Sephadex A-50 gel adsorption: add DEAE Sephadex A-50 gel to the supernatant obtained in step 3) for adsorption, the amount of A-50 gel is 0.0025k per kg of cryoprecipitate dry gel, A -50 dry glue soaked in swelling solution for more than 3 hours before use, and balanced with balance solution for 3 times, each time not less than 10 minutes, the adsorption pH is 6.9, and the adsorption time is 30-60 minutes. After adsorption, the supernata...
Embodiment 3
[0078] A preparation method for human blood coagulation factor VIII, comprising the steps of:
[0079] 1) Preparation of cryoprecipitate: prepare plasma cryoprecipitate;
[0080] 2) Dissolving: cryoprecipitate is dissolved to obtain a solution;
[0081] 3) Acid precipitation: adjust the pH of the solution with acetic acid solution, and lower the temperature to 16°C, collect the supernatant by centrifugation after acid precipitation, the liquid temperature is 20°C, and the liquid output speed of the centrifuge is ≤5000ml / min;
[0082] 4) DEAE Sephadex A-50 gel adsorption: add DEAE Sephadex A-50 gel to the supernatant obtained in step 3) for adsorption, the amount of A-50 gel is 0.0025 kg per kg of cryoprecipitate dry gel, A -50 dry glue is soaked in swelling solution for more than 3 hours before use, and balanced with balance solution for 3 times, each time not less than 10 minutes, the adsorption pH is 6.9, and the adsorption time is 30-60 minutes. After adsorption, the super...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com