Method for simultaneously preparing high-purity human coagulation factor VIII and human fibrinogen
A technology of human fibrinogen and human coagulation factor, applied in the direction of fibrinogen, coagulation/fibrinolytic factor, VII factor, etc., can solve the problems of FVIII white loss, human fibrinogen throwing away, etc., to reduce loss and reduce The effect of increasing labor intensity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
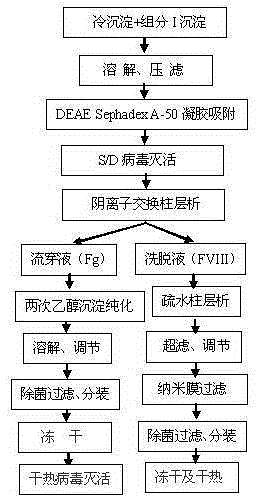
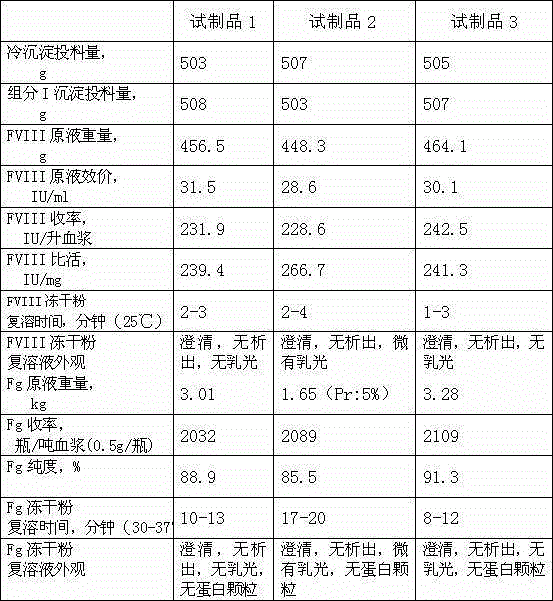
Image
Examples
Embodiment 1
[0058] 1. Prepare cryoprecipitate and component I precipitation according to the method described in step 1 of the process flow, weigh 0.5kg each of cryoprecipitate and component I precipitation, and put them into 10kg of pre-prepared dissolution buffer 1 together, and dissolution buffer 1 is composed of 0.02MTRIS-HCL, 0.06M sodium citrate, 0.02M lysine hydrochloride, 0.15MNaCL, PH6.90-7.10; heparin sodium was pre-added to 6000IU / kg in the dissolution buffer 1; the temperature was controlled at 25-30 ℃, stir for 2 hours to fully dissolve; then filter with a Supradur50P filter plate produced by Pall in series with a 0.45 μm filter element to collect the filtrate; pre-wash the filter plate and filter element with dissolution buffer 1 before filtering;
[0059] 2. Add DEAESephadexA-50 gel which was pre-swelled, cooled and balanced with the dissolution buffer 1 described in the previous step to the above filtrate. The amount of the gel added is 5.5g based on dry gel; stir slowly fo...
Embodiment 2
[0064] 1, with embodiment one;
[0065] 2. Add DEAESephadexA-50 gel that was pre-swelled, cooled and balanced with the dissolution buffer 1 described in the previous step to the above filtrate. The amount of the gel added is 11g based on dry gel, pH6.90-7.10; slowly stir for at least 45 minutes , after standing for 30 minutes; then filter with filter cloth and collect the filtrate;
[0066] 3, with embodiment one;
[0067] 4. Put the filtrate after virus inactivation above on the CaptoDEAE column. The column is fully equilibrated with equilibration buffer 1 in advance. The composition of equilibration buffer 1 is 0.02MTRIS-HCL, 0.06M sodium citrate, 0.02M lysine hydrochloride, 0.1 MNaCl, 0.01M CaCl 2 PH7.40-7.50); use a stainless steel tank with a refrigerant jacket to collect the flow-through liquid, after loading the column, rinse the column with equilibration buffer 1, and merge the washing liquid into the flow-through liquid; then use washing buffer 1 (0.02MTRIS- HCl,...
Embodiment 3
[0070] 1~3 are with embodiment one;
[0071] 4. The above virus inactivated filtrate is put on the QSepharose4FF column. The column is fully equilibrated with equilibrium buffer 1 in advance. The composition of equilibrium buffer 1 is 0.02MTRIS-HCL, 0.05M sodium citrate, 0.04M lysine hydrochloride, 0.15 MNaCl, 0.015MCaCl 2 PH6.60-6.80); use a stainless steel tank with a refrigerant jacket to collect the flow-through liquid; after loading the column, wash the column with equilibration buffer 1, and merge the washing liquid into the flow-through liquid; then use washing buffer 1 (0.02MTRIS- HCl, 0.2M NaCl, 0.01M CaCl 2 , PH6.60-6.80) to wash the column, then use elution buffer 1 (0.02MTRIS-HCL, 1.0MNaCL, 0.003MCaCL 2 , pH6.60-6.80) to elute, filter with a 0.45μm filter element in time, and collect the filtrate; add NaCL to 1.8M, put PhenylSepharose6FF (LS), and the column is fully equilibrated with equilibrium buffer 2 in advance, and the composition of equilibrium buffer 2 is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com