Preparation method of cold sediment and application of cold sediment to production of human coagulation factor VIII
A technology of human coagulation factor and cryoprecipitation, which is applied in the fields of biopharmaceuticals and blood products, can solve the problems of attenuation of cryoprecipitated human coagulation factor VIII activity and long plasma melting time, so as to protect the activity of factor VIII, reduce the melting time and control the activity Attenuation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
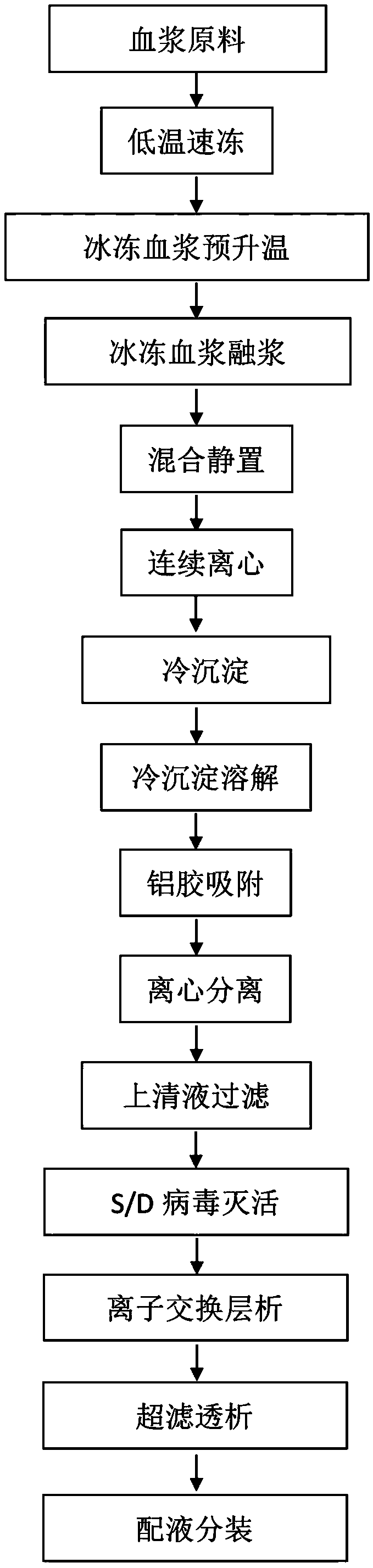
[0033] [embodiment 1] the preparation of cryoprecipitate of the present invention
[0034] (1) Quick-freezing of fresh human plasma: After a single bag of 600g fresh plasma is collected, it is completely frozen within 30 minutes with a blower-type continuous quick-freezing device at -30°C until there is no fluid liquid, and stored in a freezer below -20°C to ensure that the plasma activities of various protein components.
[0035] (2) Transportation of frozen human plasma raw materials: Plasma transportation was carried out by a fully automatic temperature-controlled refrigerated transport vehicle, and the temperature was automatically recorded every 30 minutes during the transportation process. After the transportation arrived, it was immediately transferred to a -20°C cold storage for storage.
[0036] (3) Frozen human plasma raw material thawing: Take 1600 bags of frozen human plasma raw material stored in a -20°C freezer for one year, and place the frozen human plasma raw ...
Embodiment 2
[0040] [embodiment 2] the preparation of cryoprecipitate of the present invention
[0041] (1) Quick-freezing of fresh human plasma: After a single bag of 600g fresh plasma is collected, it is completely frozen within 30 minutes with a blower-type continuous quick-freezing device at -30°C until there is no fluid liquid, and stored in a freezer below -20°C to ensure that the plasma activities of various protein components.
[0042](2) Transportation of frozen human plasma raw materials: Plasma transportation was carried out by a fully automatic temperature-controlled refrigerated transport vehicle, and the temperature was automatically recorded every 30 minutes during the transportation process. After the transportation arrived, it was immediately transferred to a -20°C cold storage for storage.
[0043] (3) Frozen human plasma raw material thawing: Take 3200 bags of frozen human plasma raw material stored in a -20°C freezer for one year, and place the frozen human plasma raw m...
Embodiment 3
[0047] [Example 3] The cryoprecipitate prepared by the present invention is used to produce human coagulation factor VIII
[0048] With cryoprecipitate prepared by the present invention as raw material, human coagulation factor VIII is produced as follows:
[0049] Cryoprecipitate dissolved (water for injection: 1~5L / kg cryoprecipitate, heparin sodium injection: 1~15IU / g water for injection, pH: 7.0±0.5, temperature: 15~25℃);
[0050] Aluminum glue adsorption (1~3%Al(OH) 3 Gel: 108g~1080g / kg cryoprecipitate), adjust pH to 6.5±0.5 with 0.1mol / L acetic acid, and centrifuge (outlet temperature: 10~25℃);
[0051] Filter the supernatant and adjust the filtrate (pH: 7.0±0.5, temperature: 15-25°C);
[0052] S / D virus inactivation (1%±0.3%Tween-80, 0.3%±0.06%TNBP, 25~26℃, 8h);
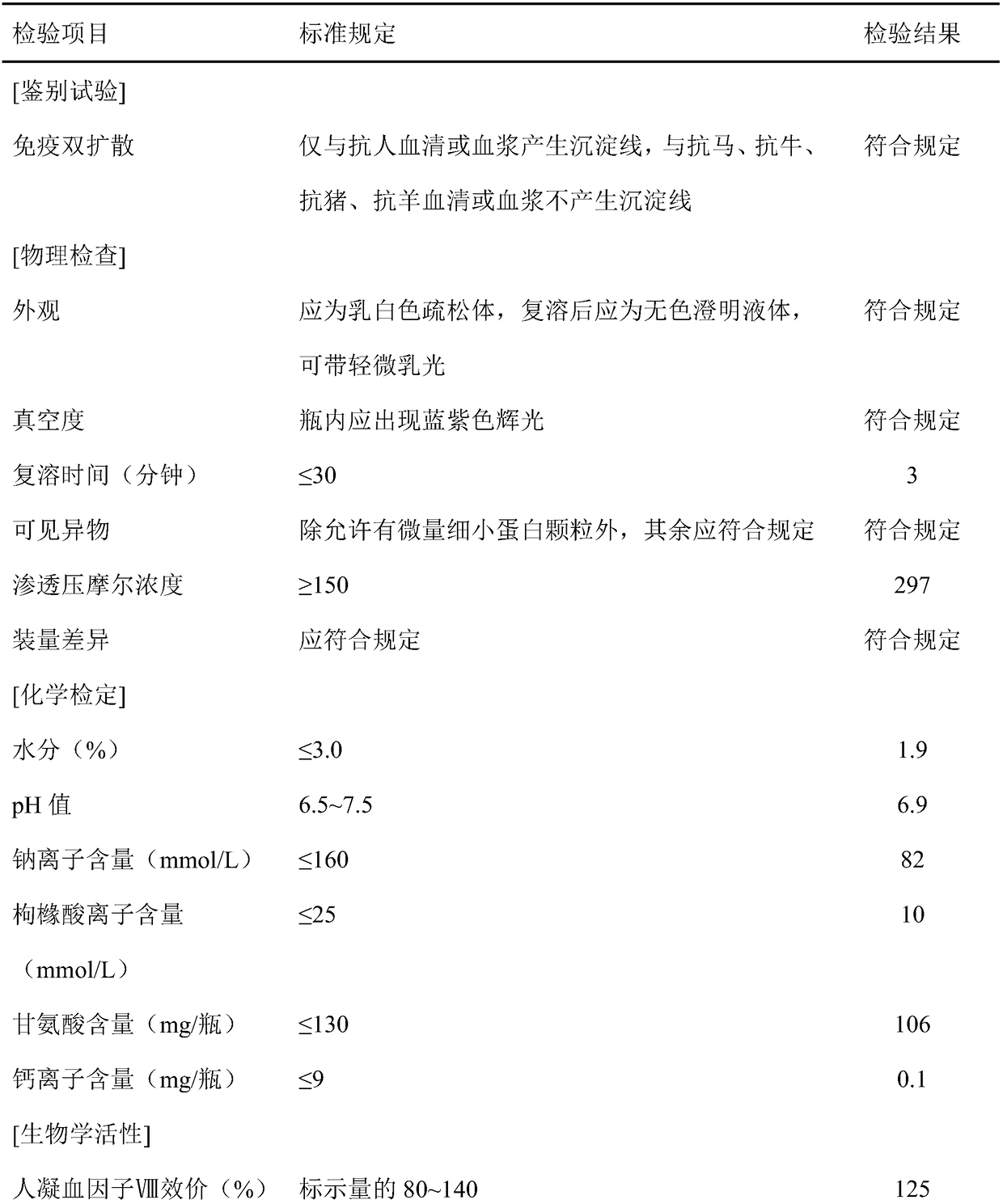
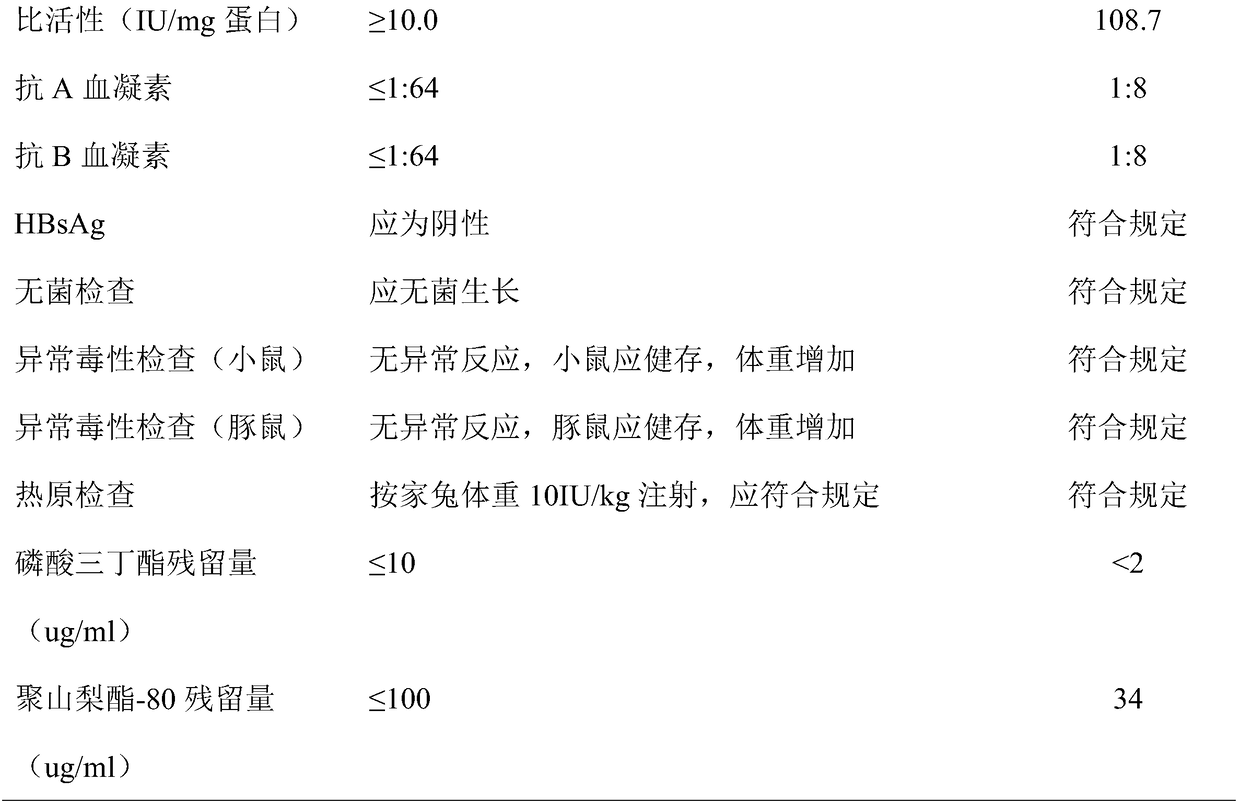
[0053] After filtration, ion exchange chromatography, ultrafiltration, dialysis, liquid preparation and subpackaging, human blood coagulation factor VIII products are finally obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com