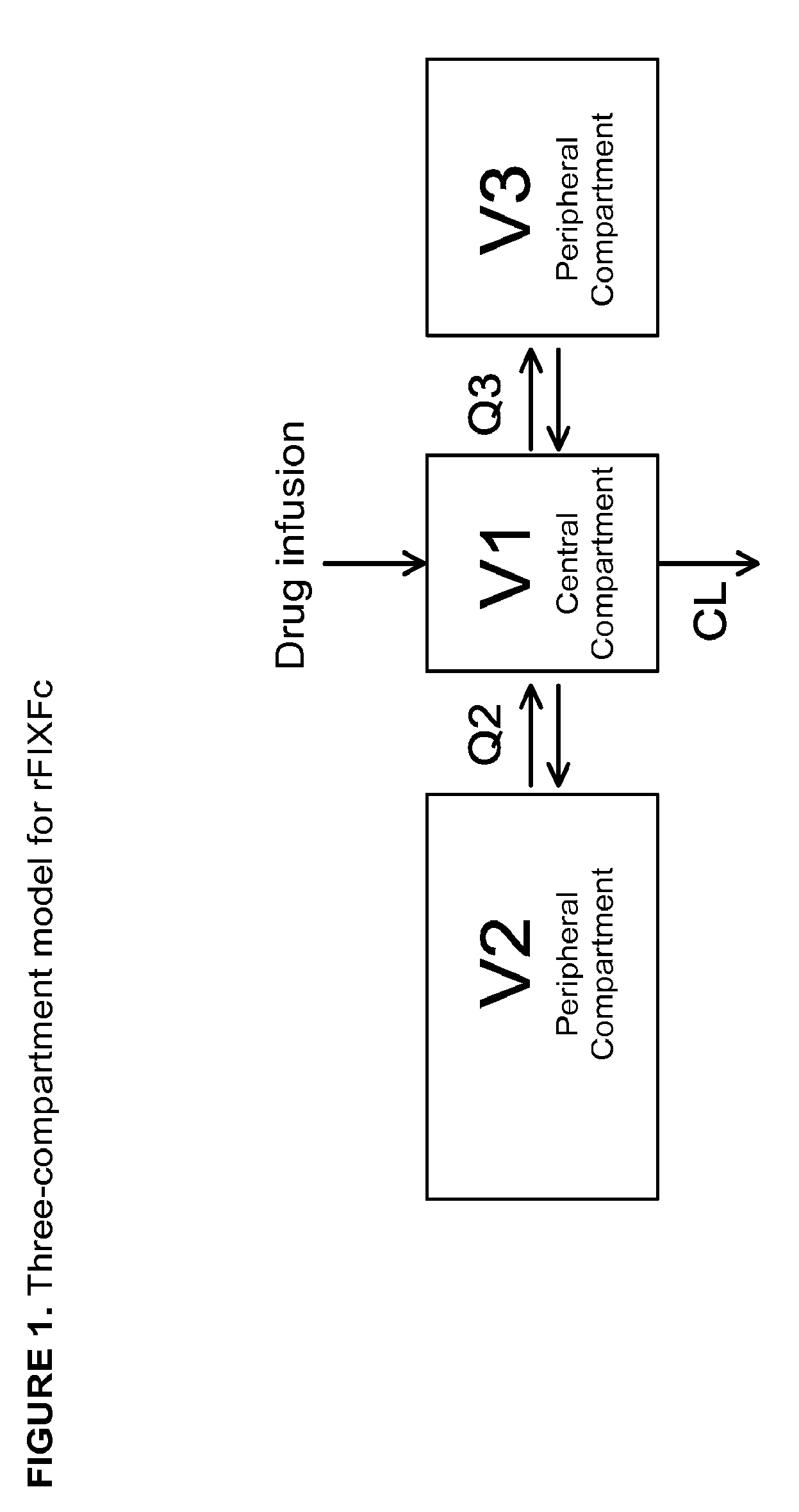
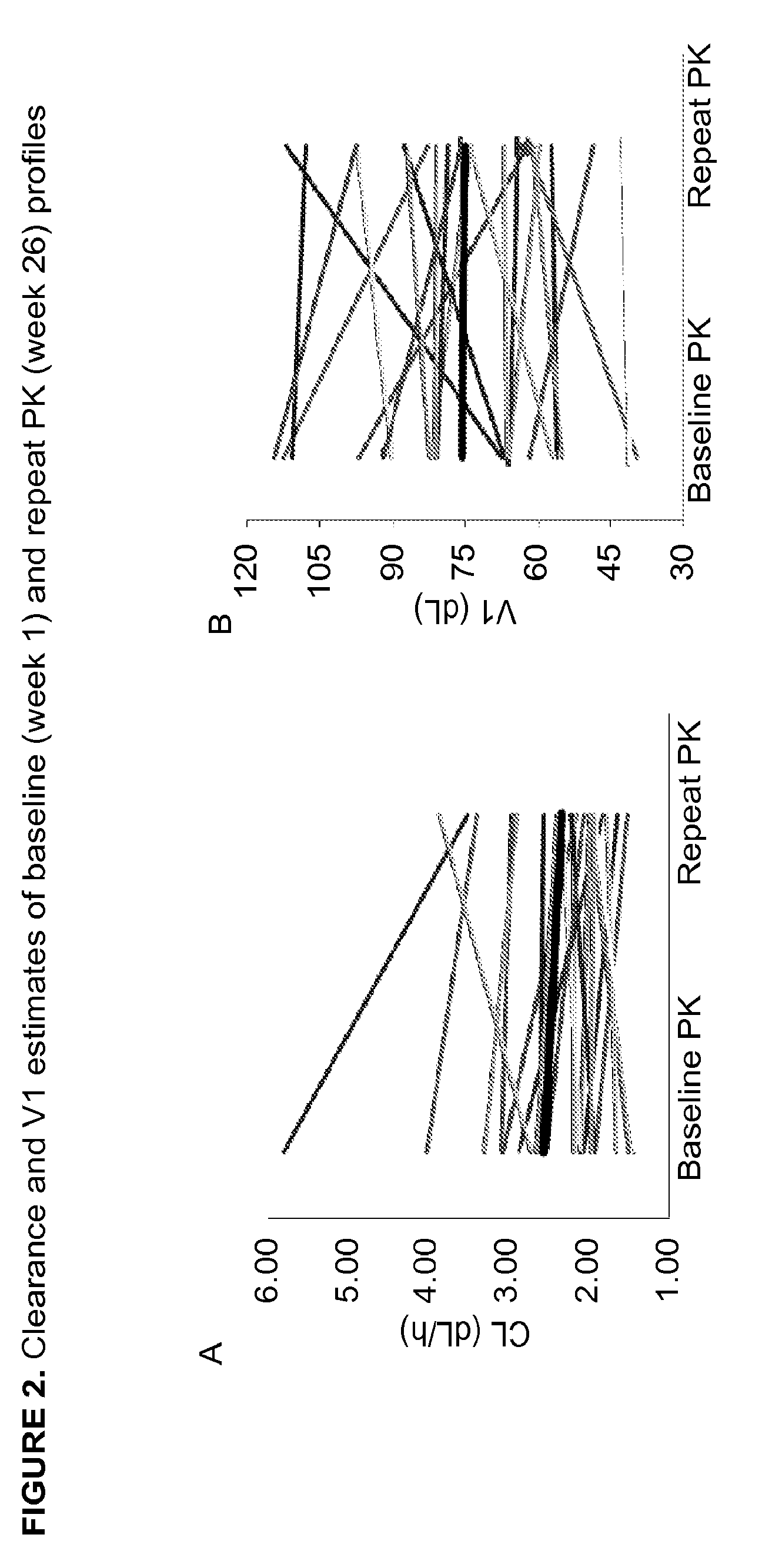
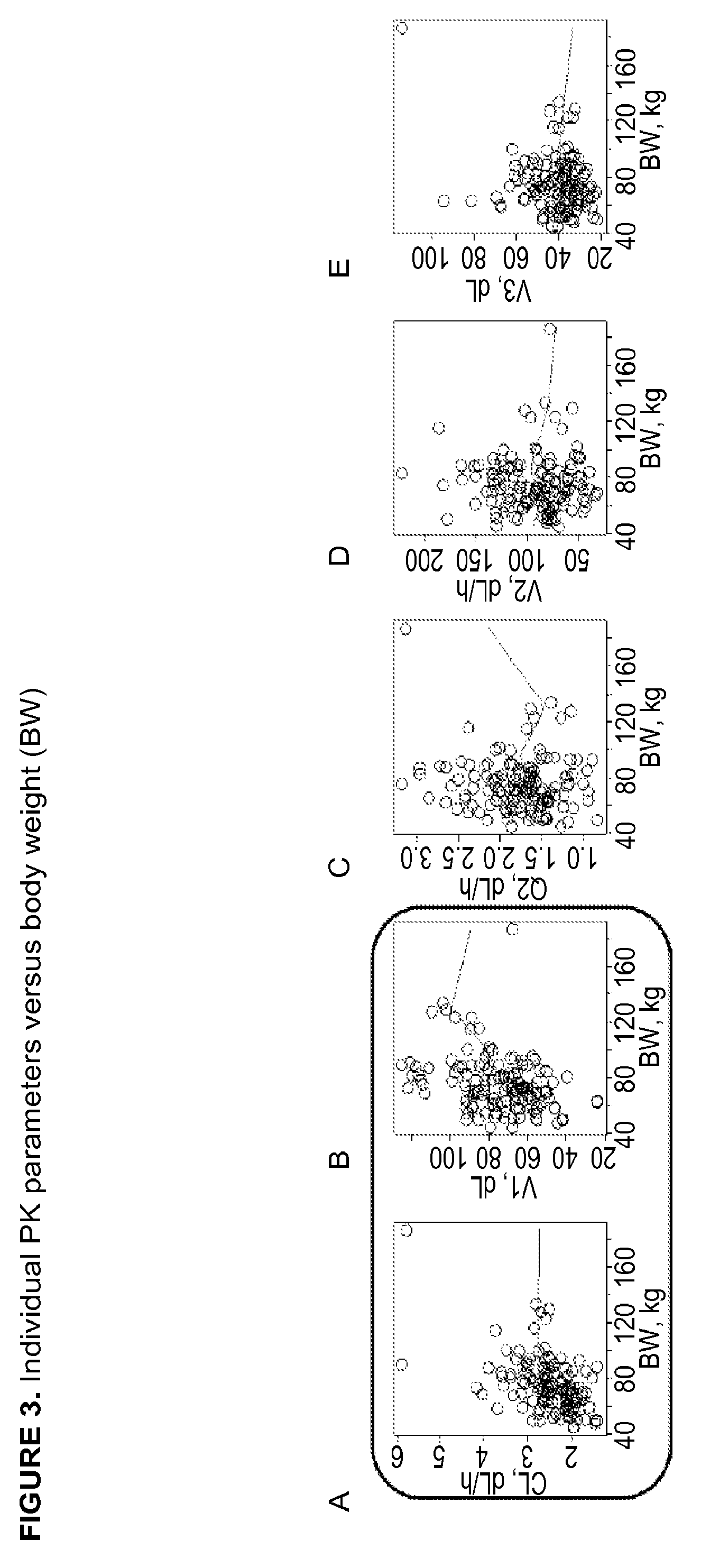
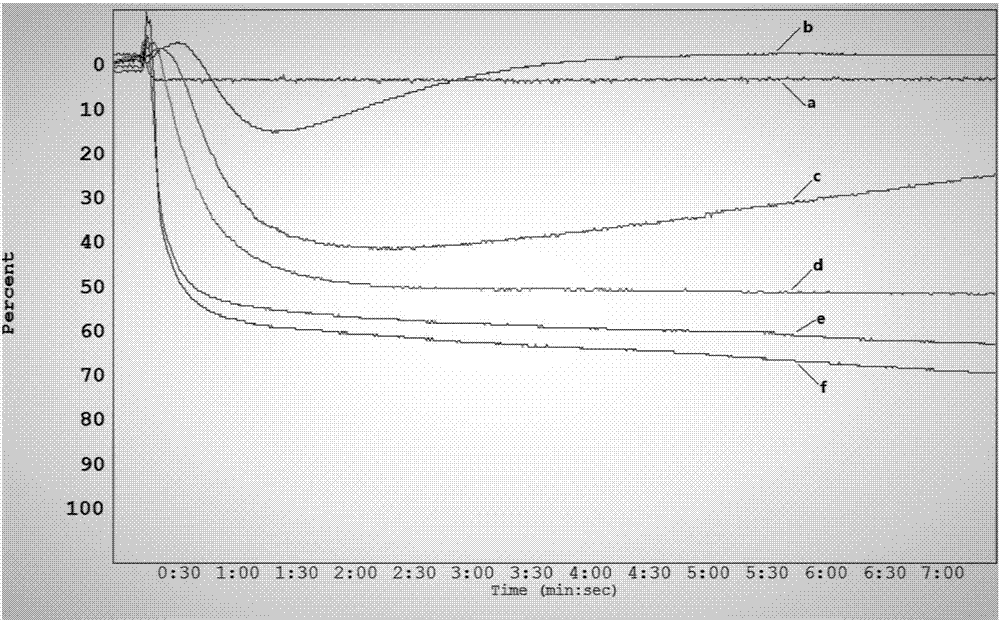
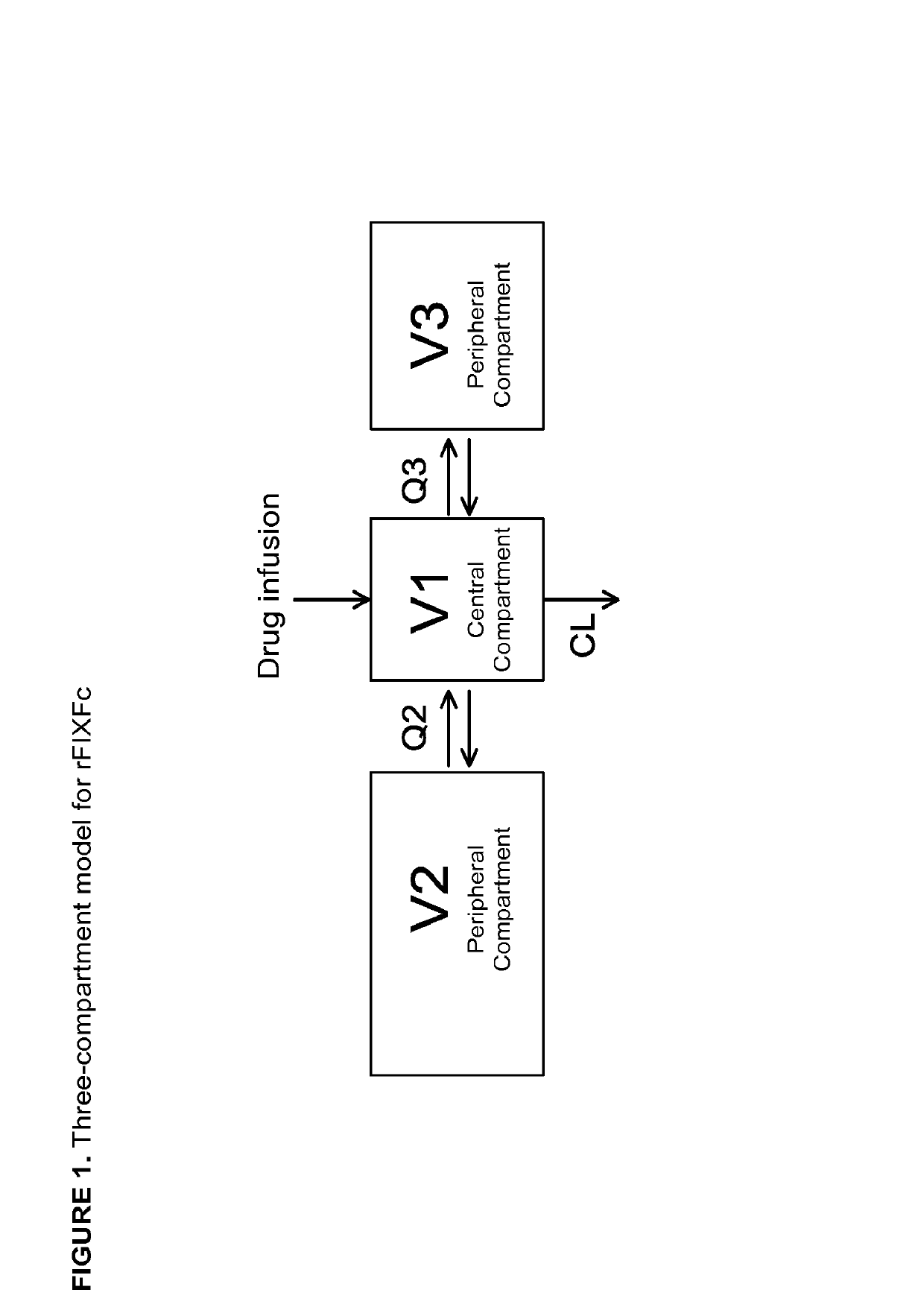
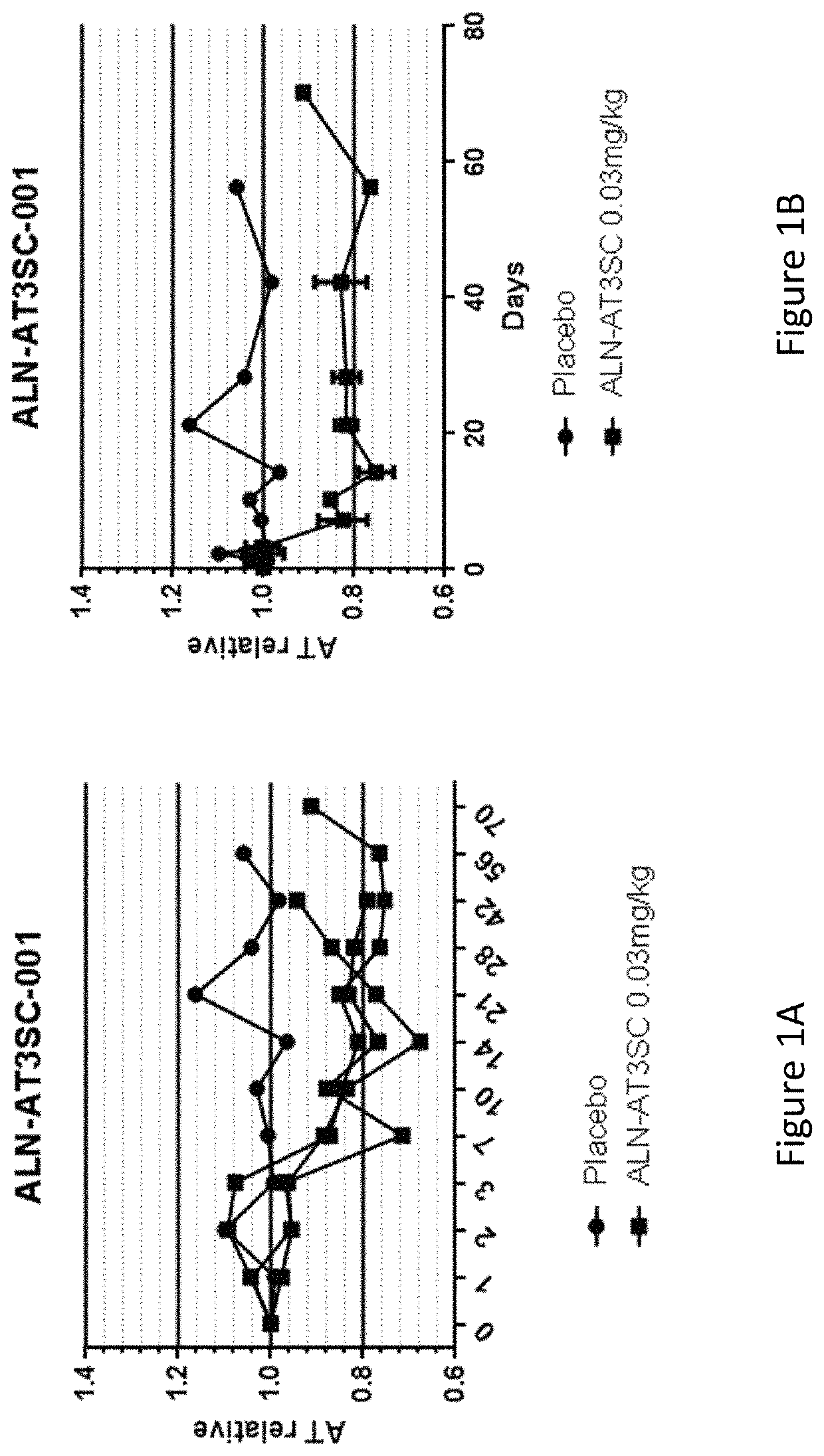
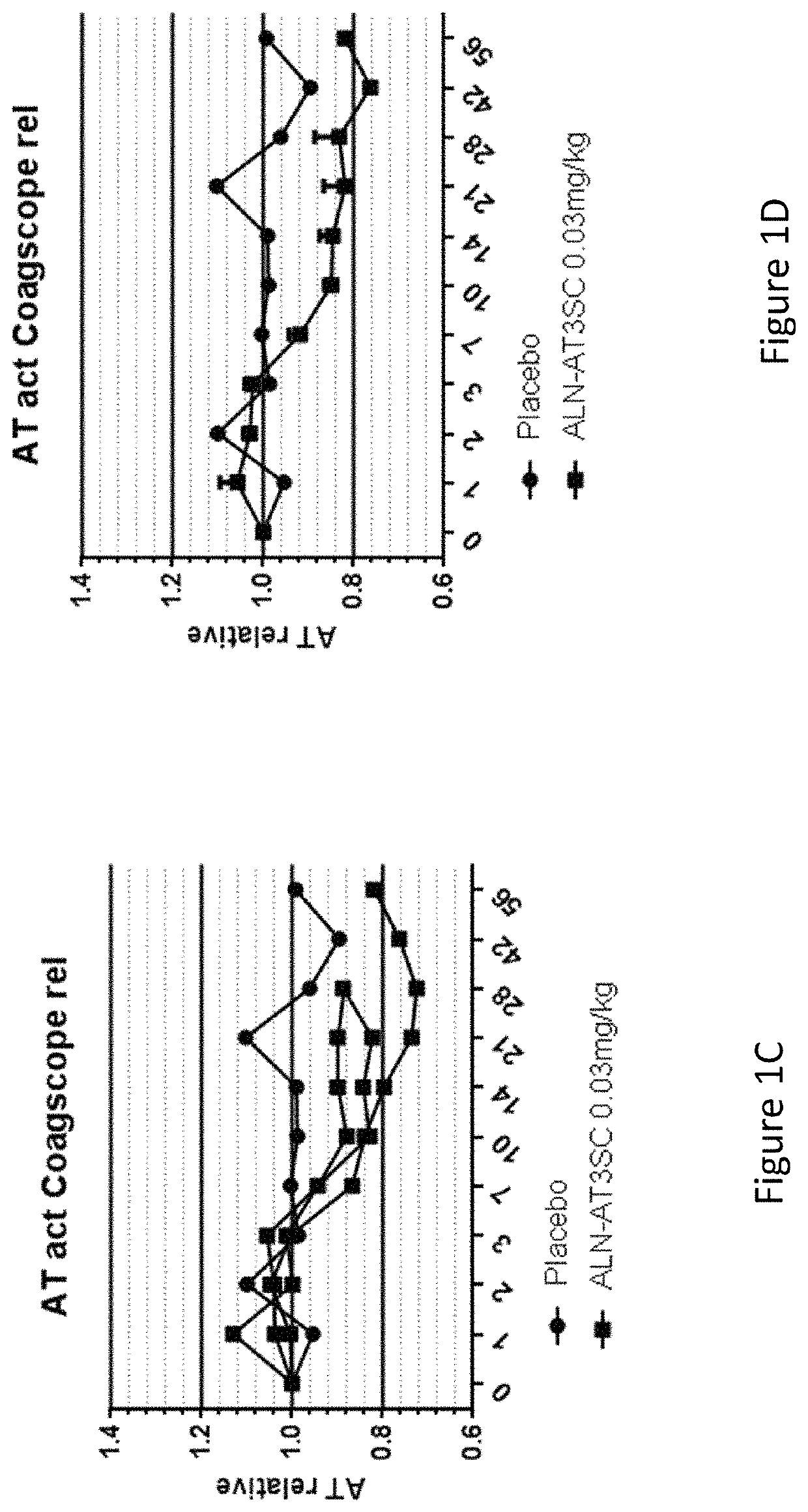
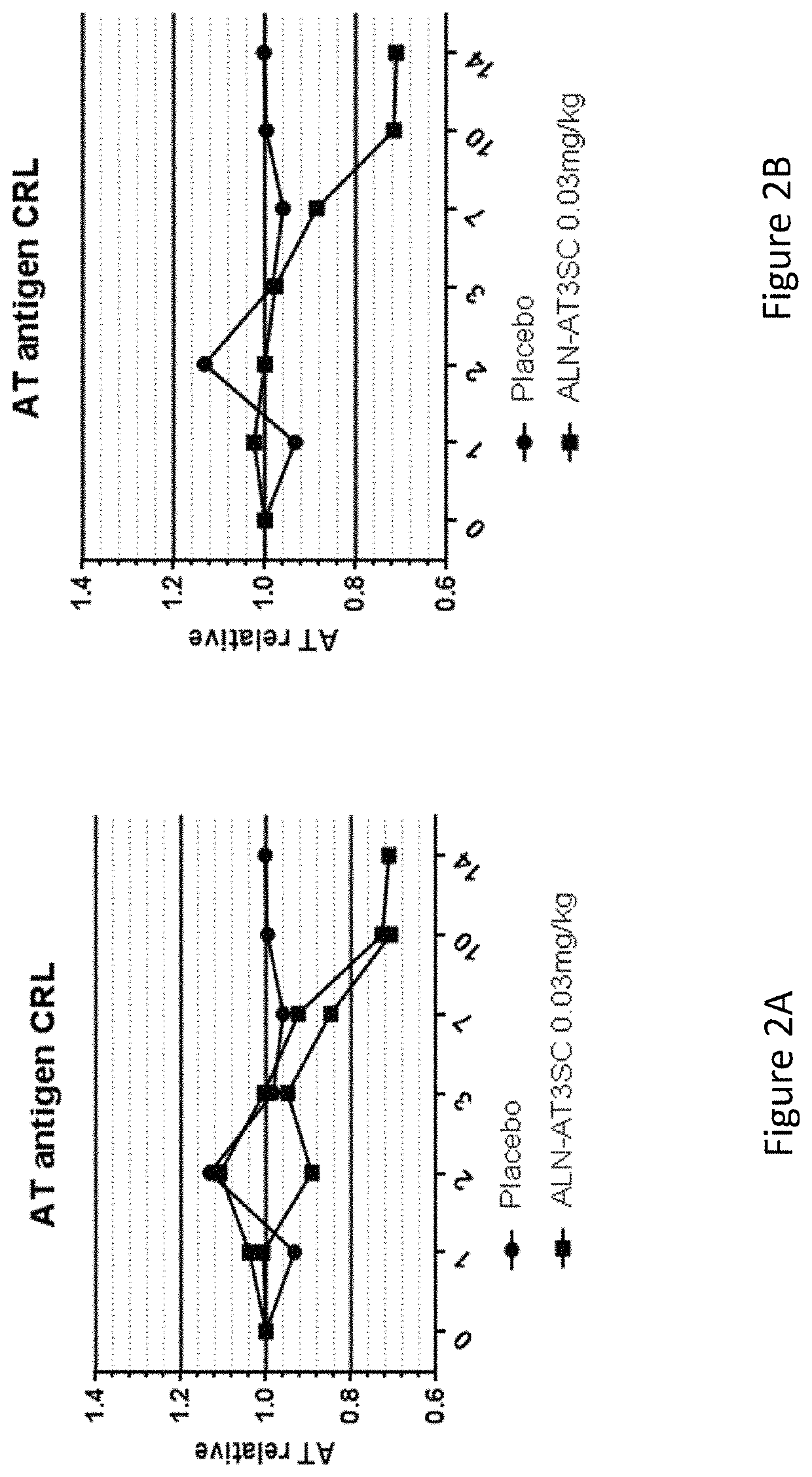
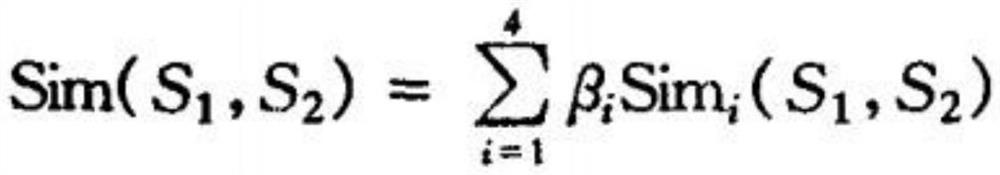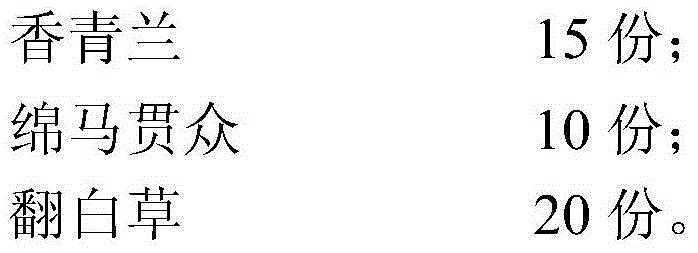Patents
Literature
48 results about "Haemorrhagic disorders" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
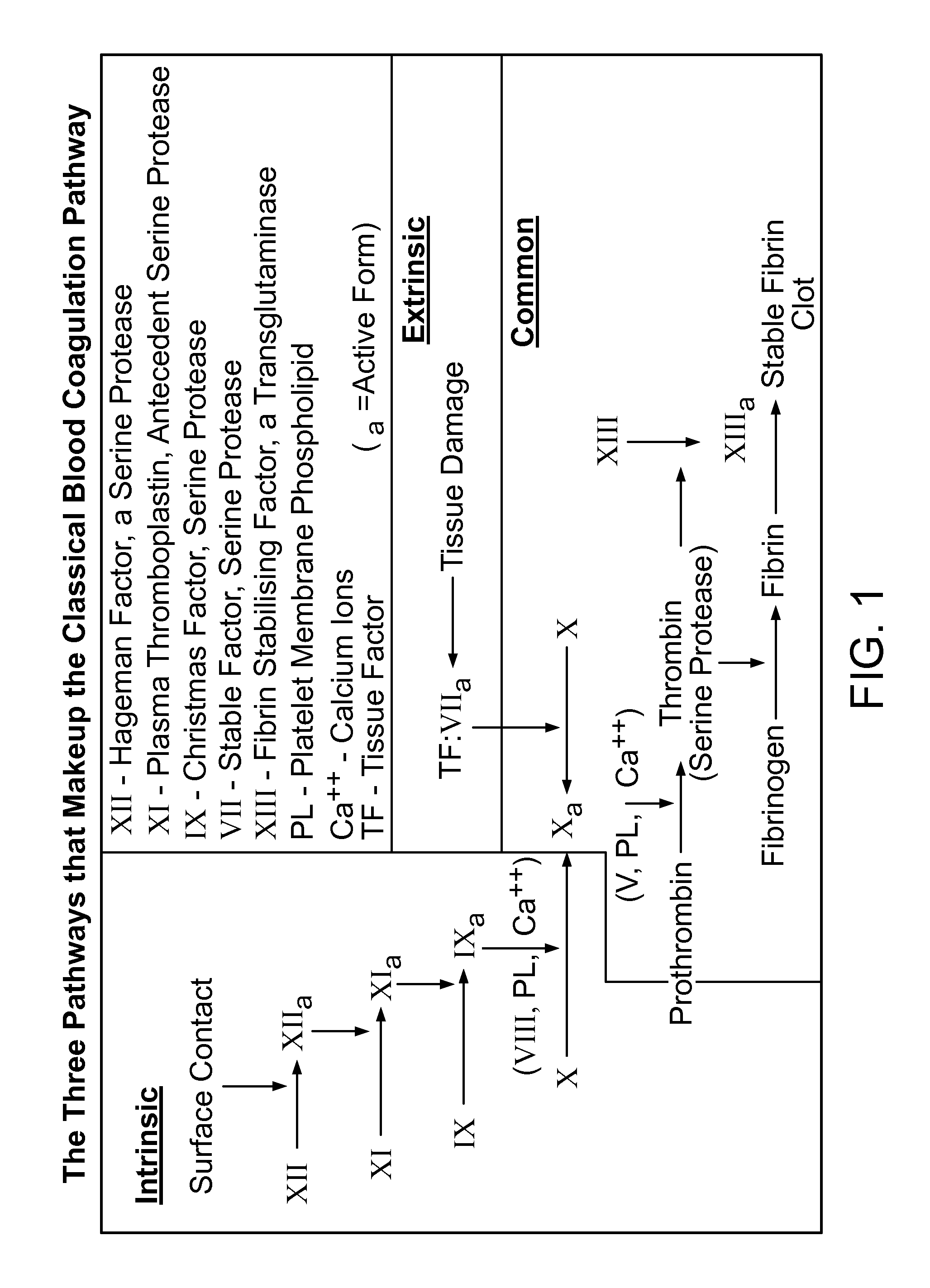
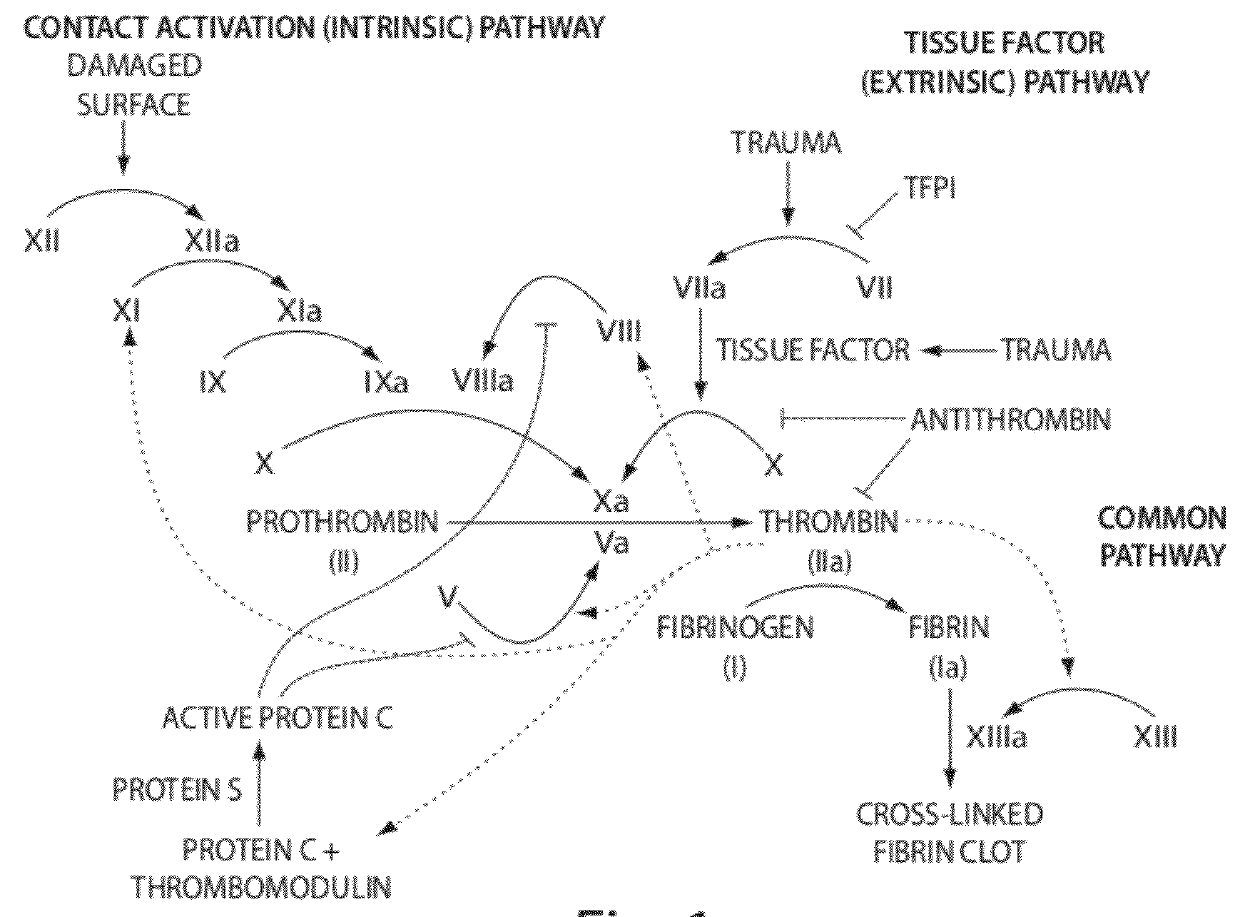
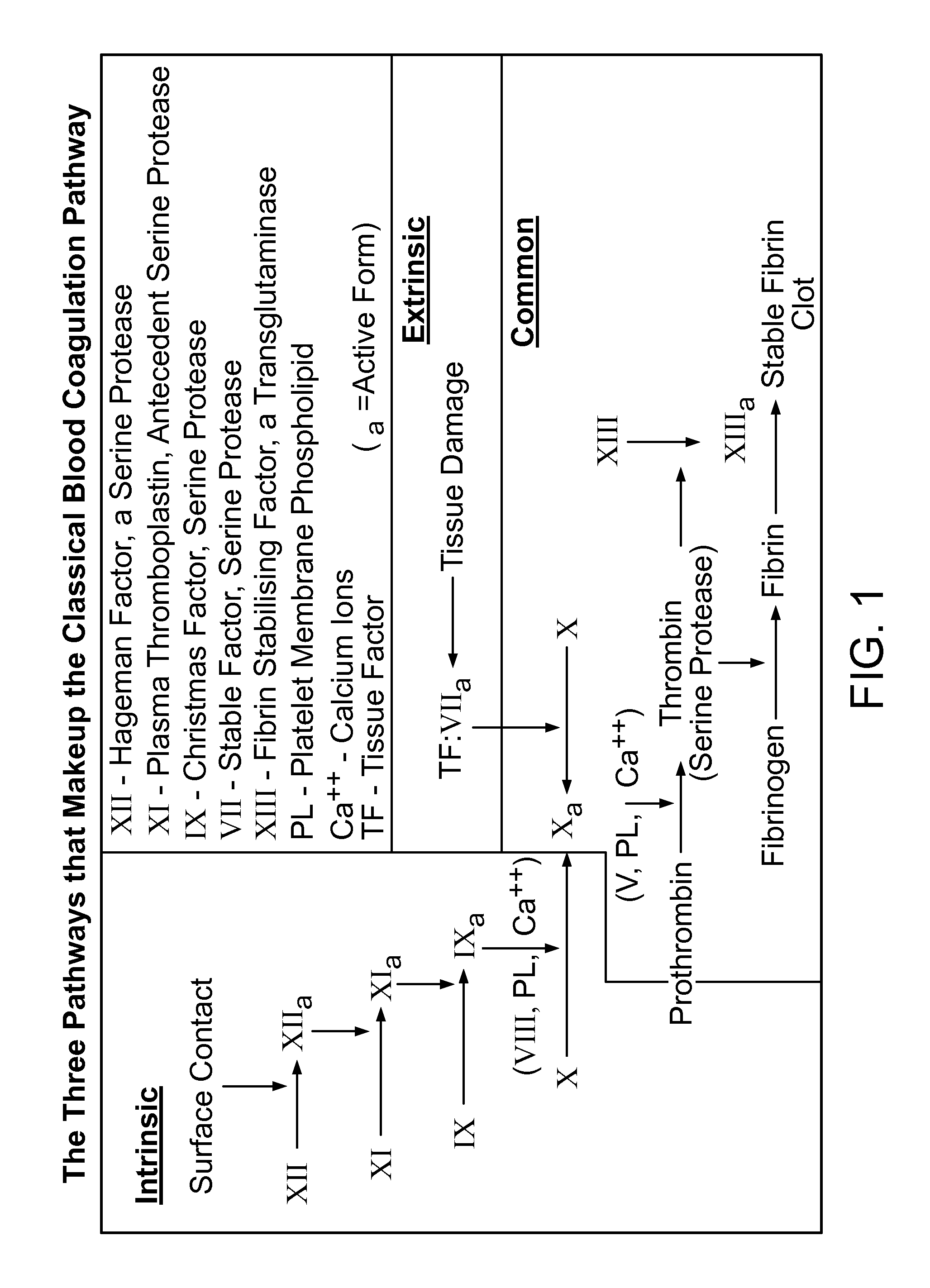
Hemorrhagic disorder: spontaneous or near spontaneous bleeding caused by a defect in clotting mechanisms (blood coagulation disorders) or another abnormality causing a structural flaw in the blood vessels (vascular hemostatic disorders).
Antimicrobial barriers, systems, and methods formed from hydrophilic polymer structures such as chistosan
An antimicrobial barrier comprising a structure including a chitosan biomaterial. The antimicrobial barrier can be used, e.g., (i) stanch, seal, or stabilize a site of tissue injury, tissue trauma, or tissue access; or (ii) form an anti-microbial barrier; or (iii) form an antiviral patch; or (iv) intervene in a bleeding disorder; or (v) release a therapeutic agent; or (vi) treat a mucosal surface; or (vii) combinations thereof. The structure of the antimicrobial barrier may be densified by compression.
Owner:HEMCON MEDICAL TECH
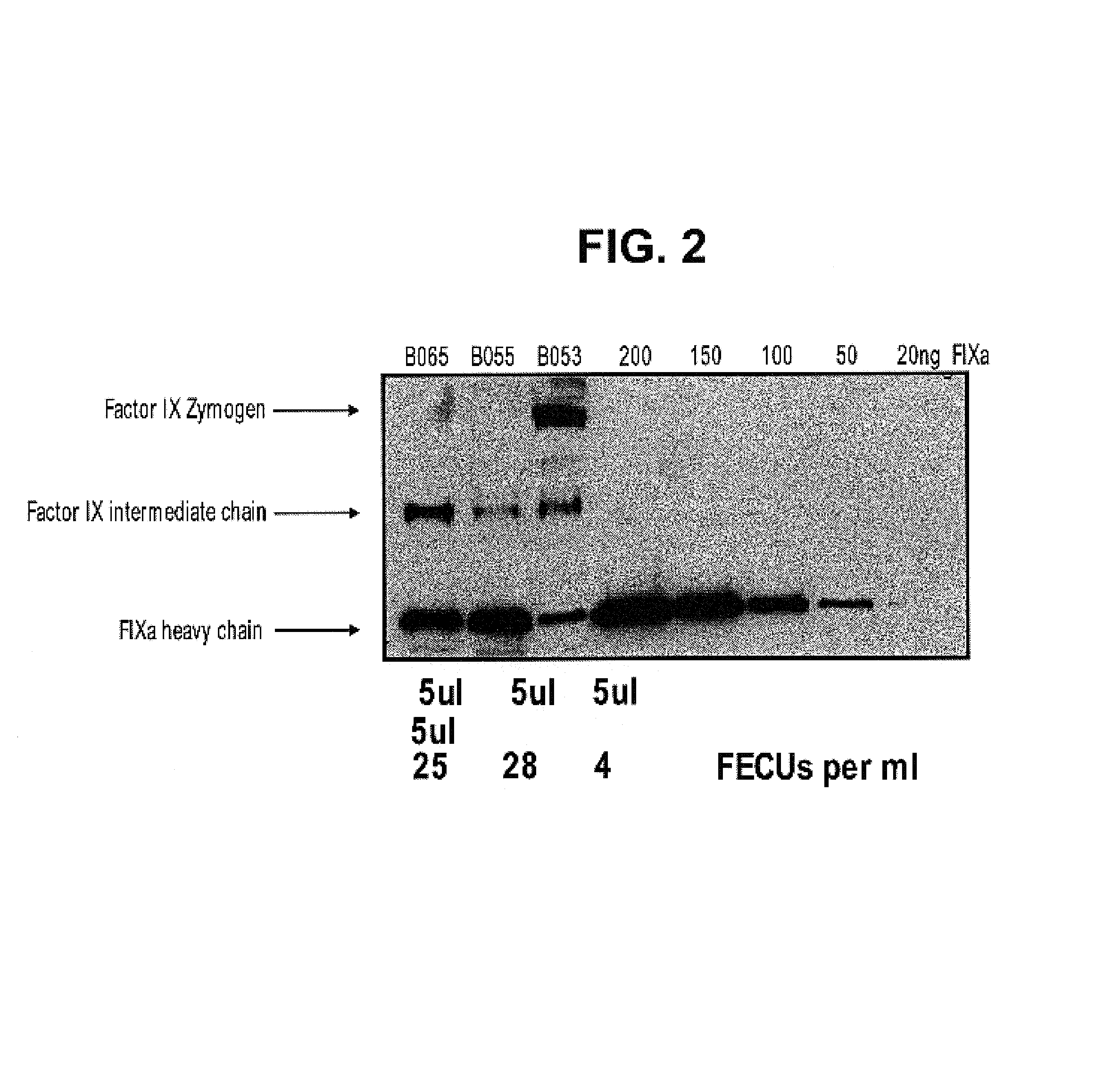
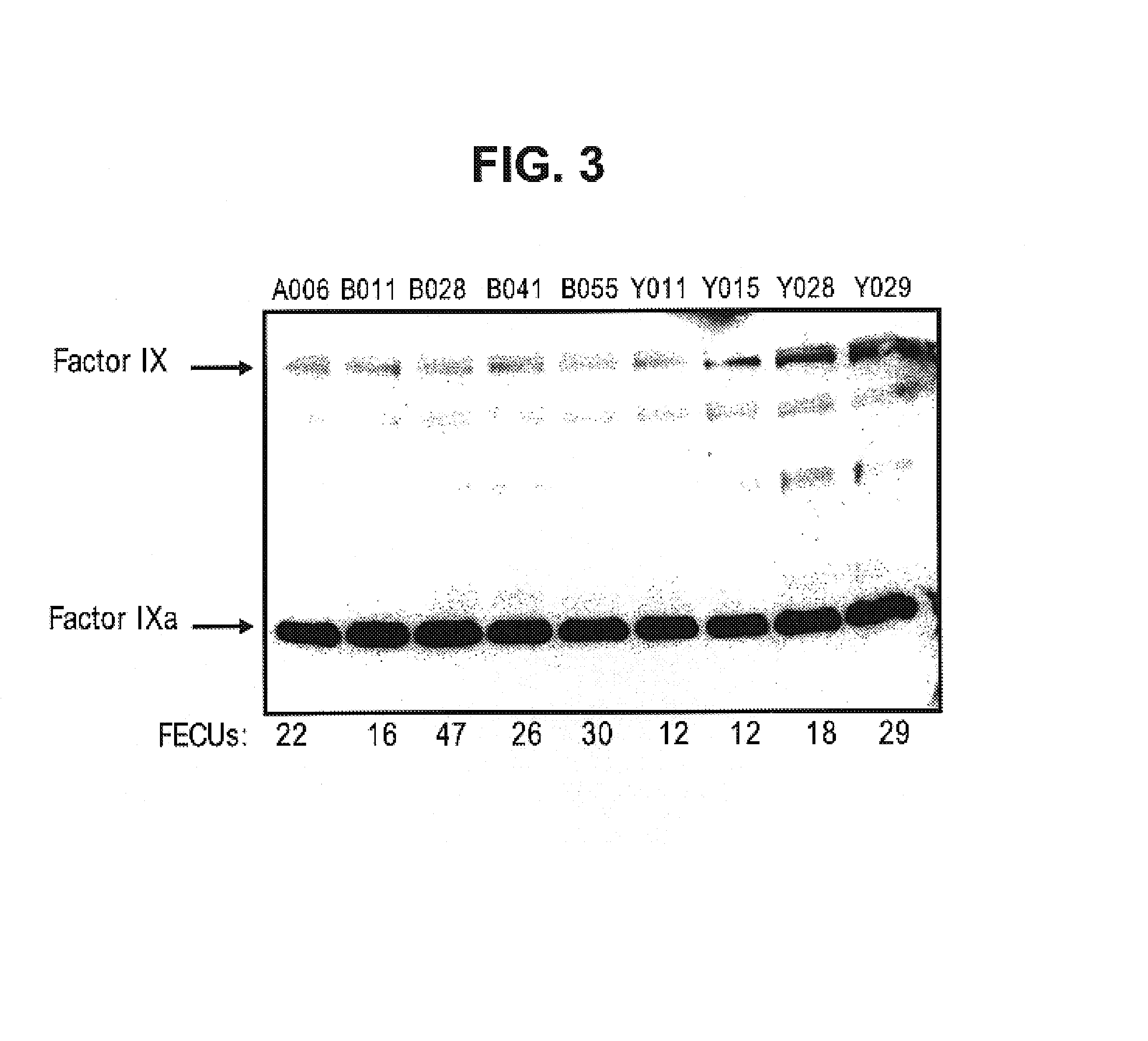
Factor IXa for the treatment of bleeding disorders
Owner:BAXALTA GMBH
Methods for treating bleeding disorders
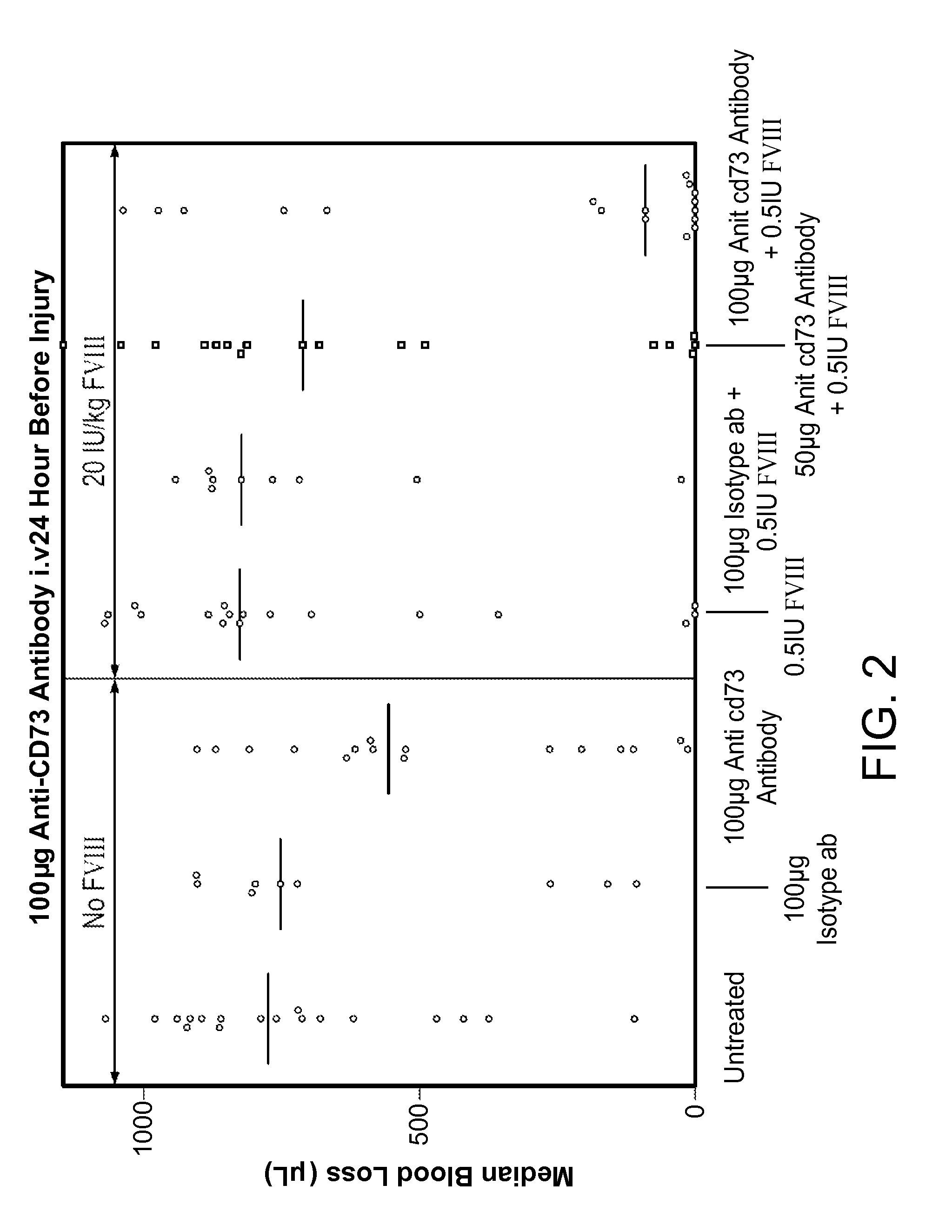
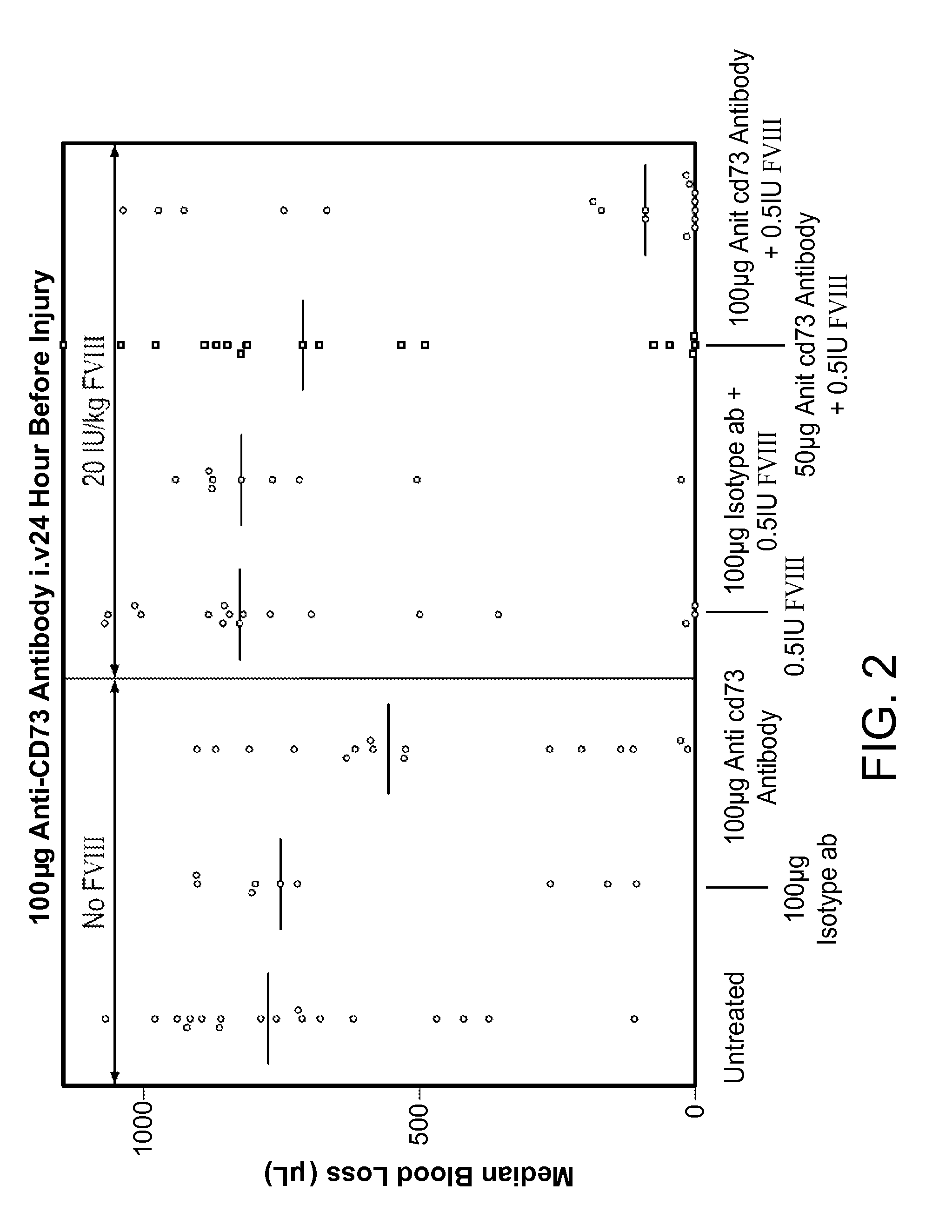
InactiveUS9090697B2Enhancing level of coagulationEffective conditioningImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAdenosineBiological activation
Disclosed are methods for treating bleeding disorders, such as hemophilia, in subjects in need thereof by administering an antibody that specifically binds CD73. The methods reduce production of adenosine, increase platelet activation and / or enhance the level of coagulation on the platelet surface to reduce and / or stop bleeding. In some embodiments, the methods can further include co-administering Factor VIII to treat the bleeding disorder.
Owner:BAYER HEALTHCARE LLC
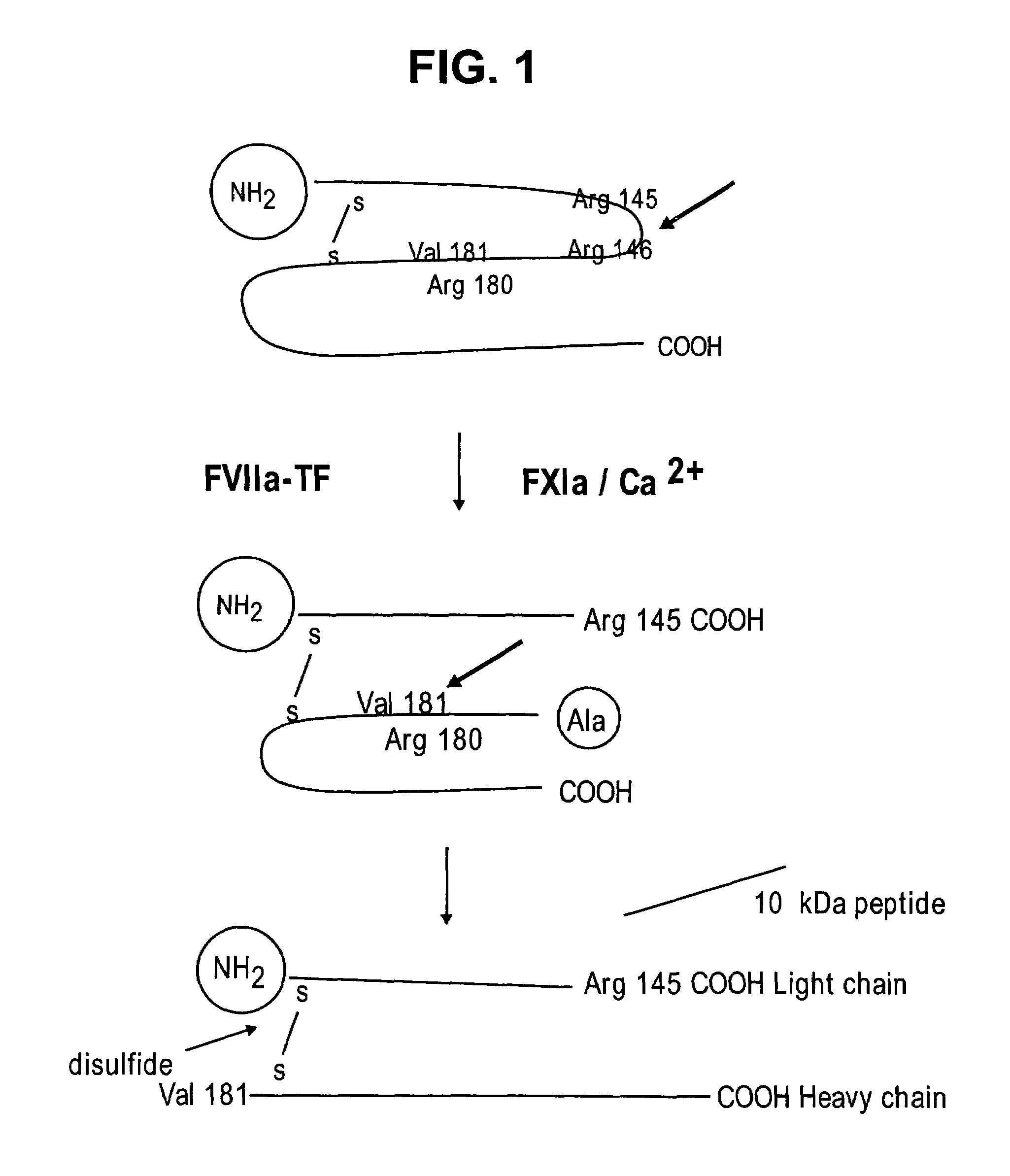
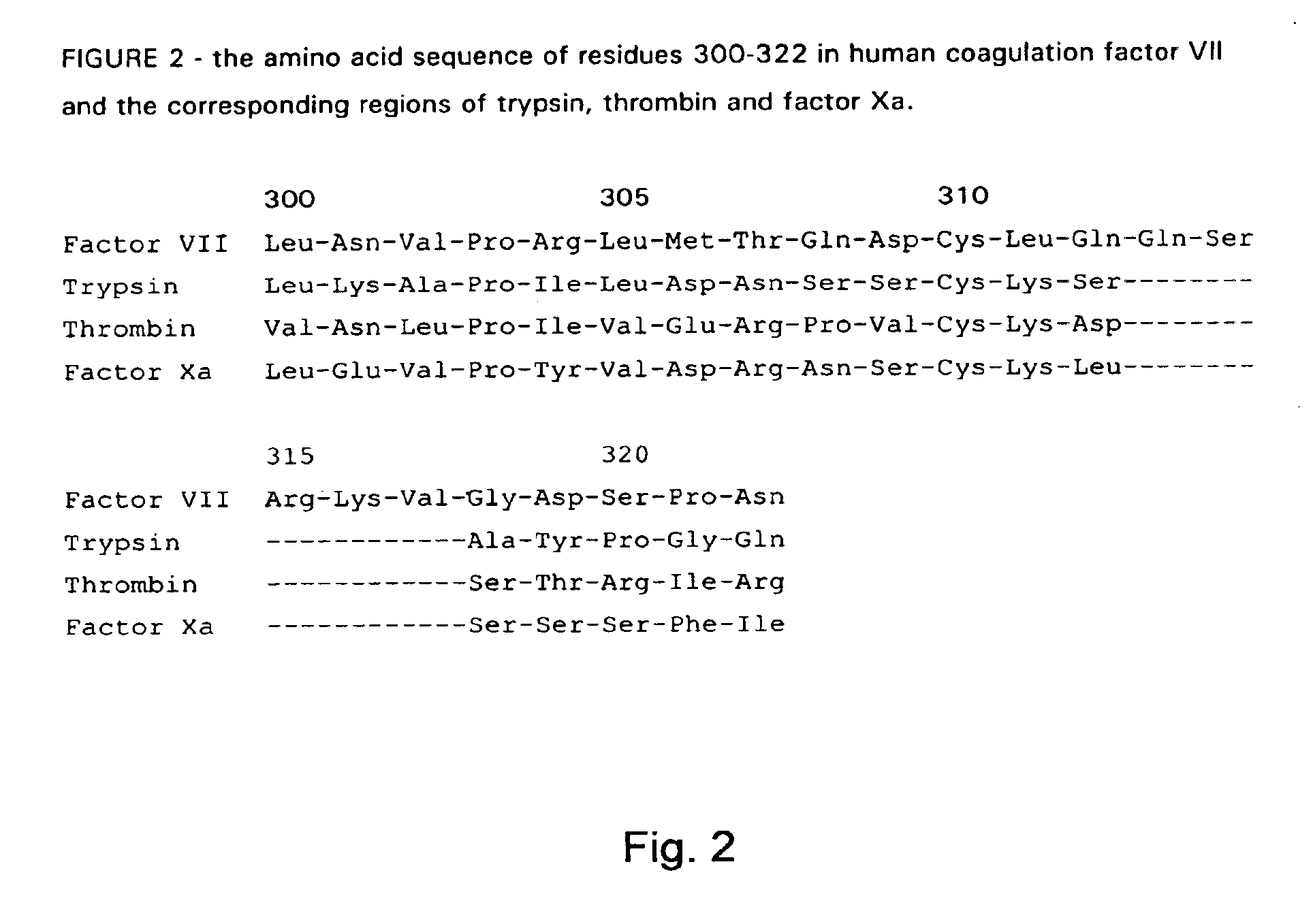
Human coagulation factor VII variants
InactiveUS6905683B2Increased tissue factor-independent activityPeptide/protein ingredientsMammal material medical ingredientsProteinase activityCoagulation system
The invention concerns novel coagulation factor VII variants, wherein the Leu residue in position 305 or the Phe residue in position 374 of SEQ ID NO 1 has been replaced by another amino acid residue which can be encoded by nucleic acid constructs and, optionally, wherein at least one other amino acid residue in the remaining positions in the protease domain has been replaced by another amino acid residue which can be encoded by nucleic acid constructs;with the proviso that the variant is not FVII(Ala305).The invention further concerns nucleic acids encoding the Factor VII variants; vectors and cells comprising the nucleic acid; methods for producing the variants; pharmaceutical compositions comprising a Factor VII variant wherein the Leu residue in position 305 or the Phe residue in position 374 of SEQ ID NO 1 has been replaced by another amino acid residue which can be encoded by nucleic acid constructs and, optionally, wherein at least one other amino acid residue in the remaining positions in the protease domain has been replaced by another amino acid residue which can be encoded by nucleic acid constructs; use of the variants for producing a medicament for treatment or prophylaxis of bleeding disorders or enhancement of the coagulation system; and methods of treatment.
Owner:NOVO NORDISK AS
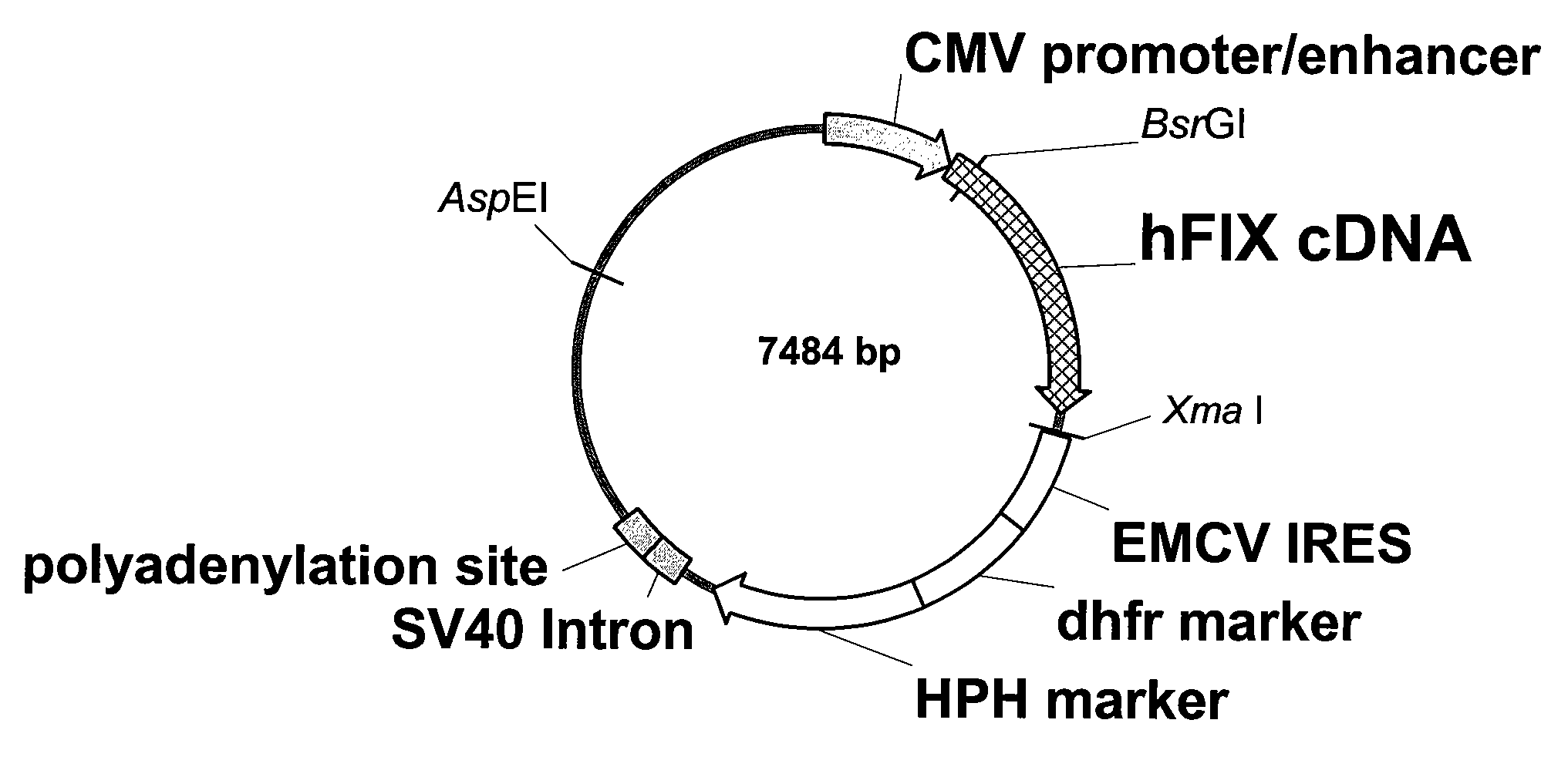
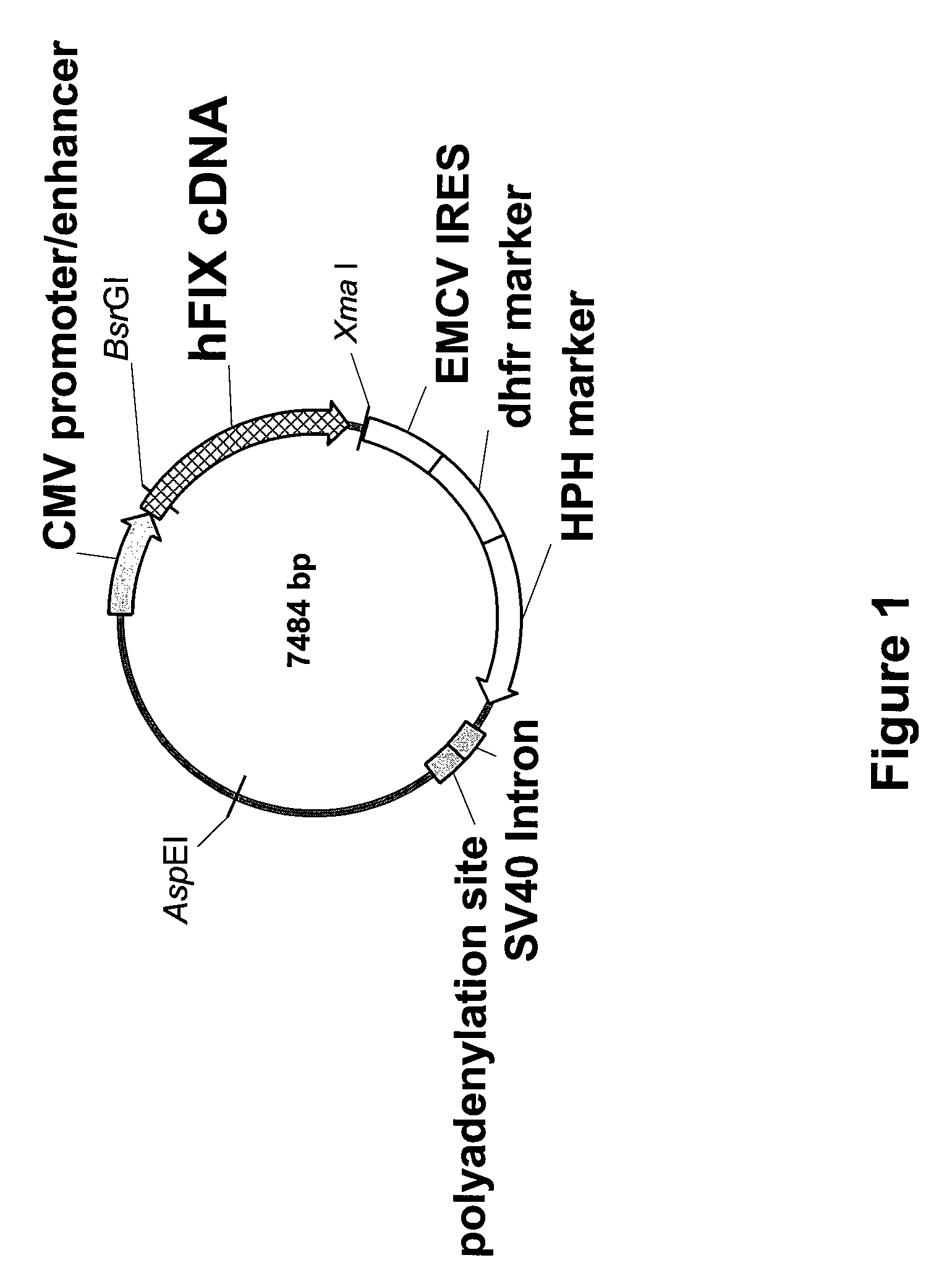
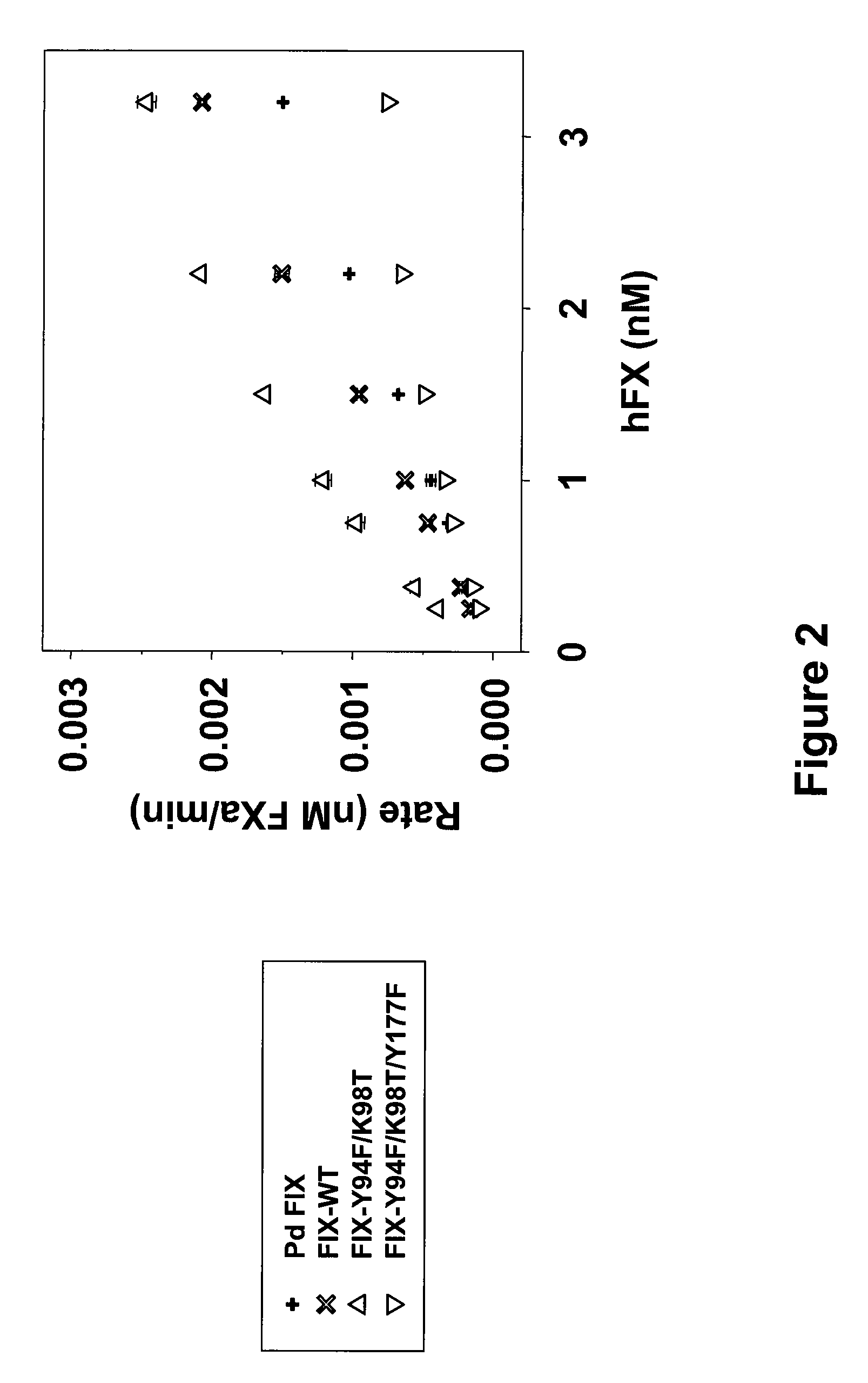
FIX-Mutant Proteins for Hemophilia B Treatment
InactiveUS20080214462A1Improved clot activityHigh activityPeptide/protein ingredientsMammal material medical ingredientsHEK 293 cellsDisease
The present invention relates to recombinant blood coagulation factor IX (rFIX) mutants having improved FIX clotting activity. Three full length FIX proteins with combinations of mutations of amino acids important for functional activity of FIX and FIX wild type were cloned and expressed in HEK 293 cells. The proteins were tested by an activated partial thromboplastin time (aPTT) assays in FIX-depleted plasma. Two mutant proteins had increased specific FIX activity. Furthermore, a pre-activated FIX protein had an increased activity in FIX-depleted plasma. Therefore these FIX mutants can be used for the treatment of FIX associated bleeding disorders.
Owner:BAXTER INT INC +1
Method for detecting whole-blood sample coagulation item using magnetic-bead method
InactiveCN1936580AEasy to masterAccurate reflectionBiological testingMagnetic beadBlood coagulations
One kind blood samples detection method using magnetic beads, completed with the following steps: (1) Get fresh clinical samples; (2)Put these specimen on coagulation analyzer, use magnetic beads and special adjustment reagents aiming at the whole blood specimens, measure four coagulations: Activated partial thromboplastin time (APTT), Prothrombin time (PT), Prothrombin time (TT), Fibrinogen (FIB). The advantages of this invention are: 1.The method is simple, time-saving and easy to technical staff. 2. Reduce costs and save centrifuges and other equipment, reduce errors; 3. It is efficient and suitable for the battlefield, natural disasters and remote mountainous areas, medical teams. Particularly, it's suitable for hemorrhagic diseases and natural disasters (such as hemophilia, etc.). 4. Save specimen volume. 5. Reflect the level of specimens of blood coagulation preciously and accurately.
Owner:MEIDE TAIPINGYANG PRECISION INSTR MFG
Methods of using a fixed dose of a clotting factor
ActiveUS20160296607A1Low variabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseDosing regimen
The present invention provides methods of administering a clotting factor by a fixed dosing regimen; methods of reducing, ameliorating, or preventing one or more symptoms of a bleeding disease or disorder; and a kit comprising a dotting factor useful for a fixed dosing regimen. While plasma-derived and recombinant clotting factor products allow hemophilia patients to live longer and healthier, hemophilia still remains one of the most costly and complex conditions to manage.
Owner:BIOVERATIV THERAPEUTICS INC
Liquid, Aqueous Pharmaceutical Composition of Factor VII Polypeptides
InactiveUS20100166730A1Improve stabilityHeavy metal active ingredientsPeptide/protein ingredientsClotting factor deficiencyOxidation state
The present invention is directed to liquid, aqueous pharmaceutical compositions containing Factor VII polypeptides, and methods for preparing and using such compositions, as well as vials containing such compositions, and the use of such compositions in the treatment of a Factor VII-responsive syndrome, e.g., bleeding disorders, including those caused by clotting Factor deficiencies (e.g. haemophilia A, haemophilia B, coagulation Factor VII deficiency); by thrombocytopenia or von Willebrand's disease, or by clotting Factor inhibitors, and intra cerebral haemorrhage, or excessive bleeding from any cause. The preparations may also be administered to patients in association with surgery or other trauma or to patients receiving anticoagulant therapy. More particularly, the invention relates to liquid compositions stabilised against chemical and / or physical degradation. The main embodiment is represented by a liquid, aqueous pharmaceutical composition comprising a Factor VII polypeptide (i); a buffering agent (ii) suitable for keeping pH in the range of from about 4.0 to about 9.0; at least one metal-containing agent (iii), wherein said metal is selected from the group consisting of first transition series metals of oxidation state +II, except zinc, such as chromium, manganese, iron, cobalt, nickel, and copper; and a non-ionic surfactant (iv).
Owner:NOVO NORDISK HEALTH CARE AG
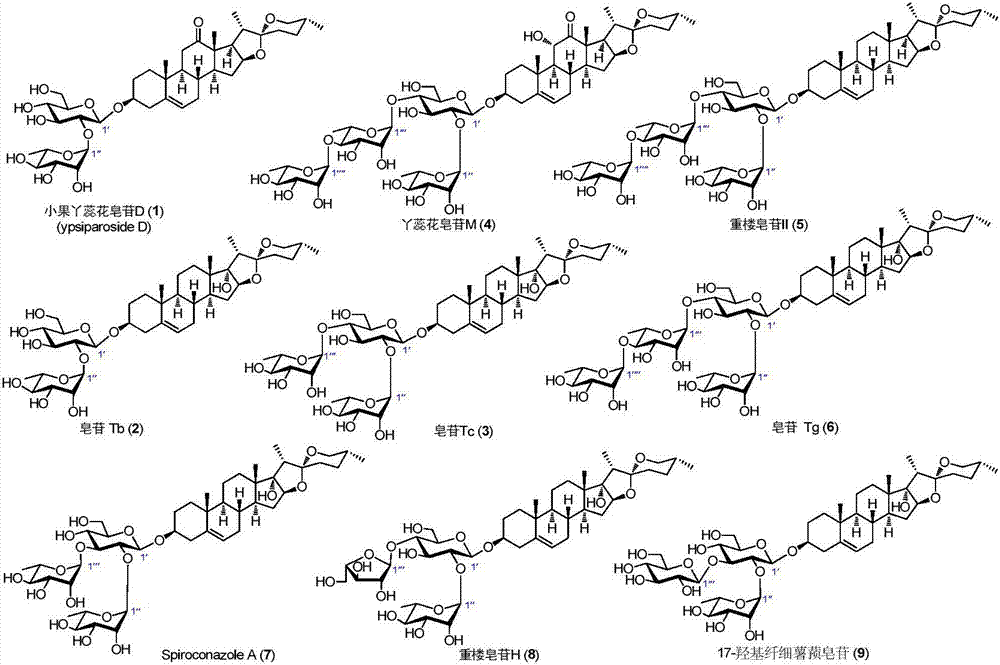
C27 spirostane-type steroidal saponin compound and pharmaceutical composition and application thereof
The invention relates to C27 spirostane-type steroidal saponins isolated from Ypsilandra, Trillium and Paris and their preparation method, as well as their application in the preparation of drugs for treating hemorrhagic diseases, and application in the preparation of drugs for treating dysfunctional uterine bleeding, and the preparation of functional daily chemical products and health products. The materials used in a method of the invention are easy to obtain, the method is simple and easily operable, biological experiments of the compound produced show that the compound can induce platelet aggregation activity and hemostatic activity significantly.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Factor IX variants with clotting activity in absence of their cofactor and their use for treating bleeding disorders
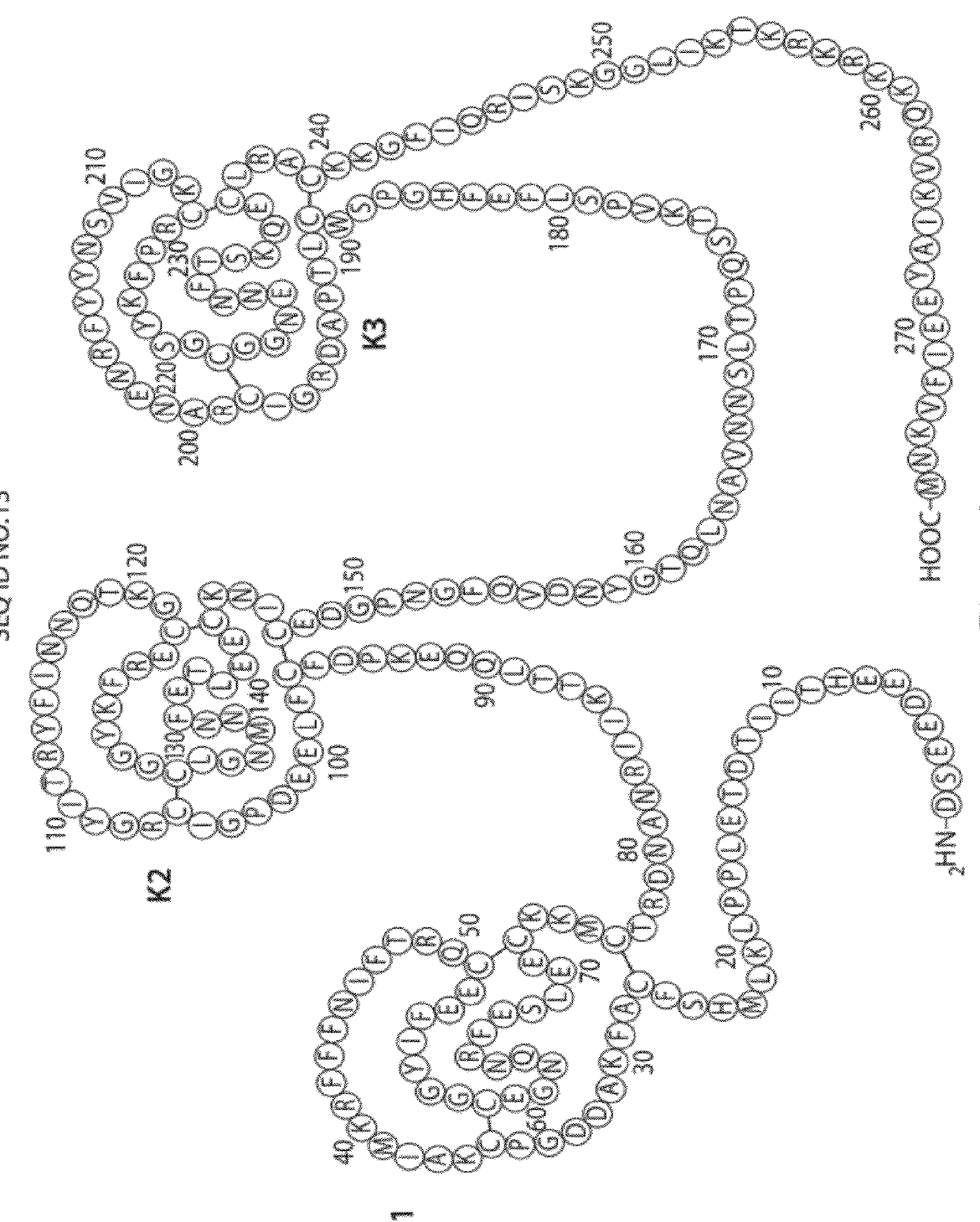
The present invention relates to variants of a vitamin K-dependent serine protease of the coagulation cascade, preferably variants of factor IX (F.IX), wherein the variant is characterized in that it has clotting activity in absence of its cofactor. The present invention furthermore relates to the use of these variants for the treatment and / or prophylaxis of bleeding disorders, in particular hemophilia A and / or hemophilia B or hemophilia caused or complicated by inhibitory antibodies to F.VIII. The present invention also relates to further variants of factor IX (F.IX) which have desired properties and can, thus be tailored for respective specific therapeutic applications.
Owner:DRK BLUTSPENDEDIENST BADEN WURTTEMBERG HESSEN GGMBH
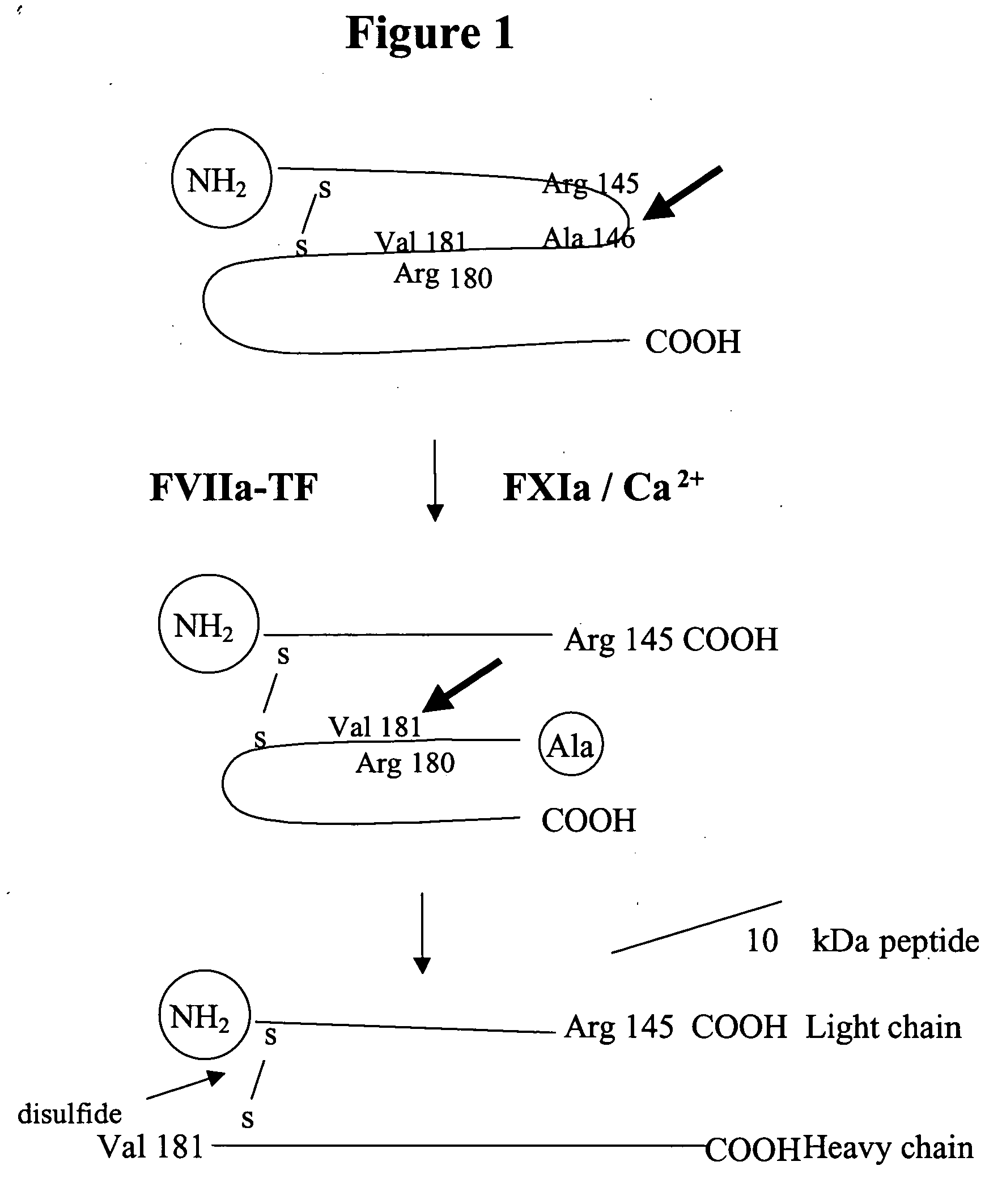
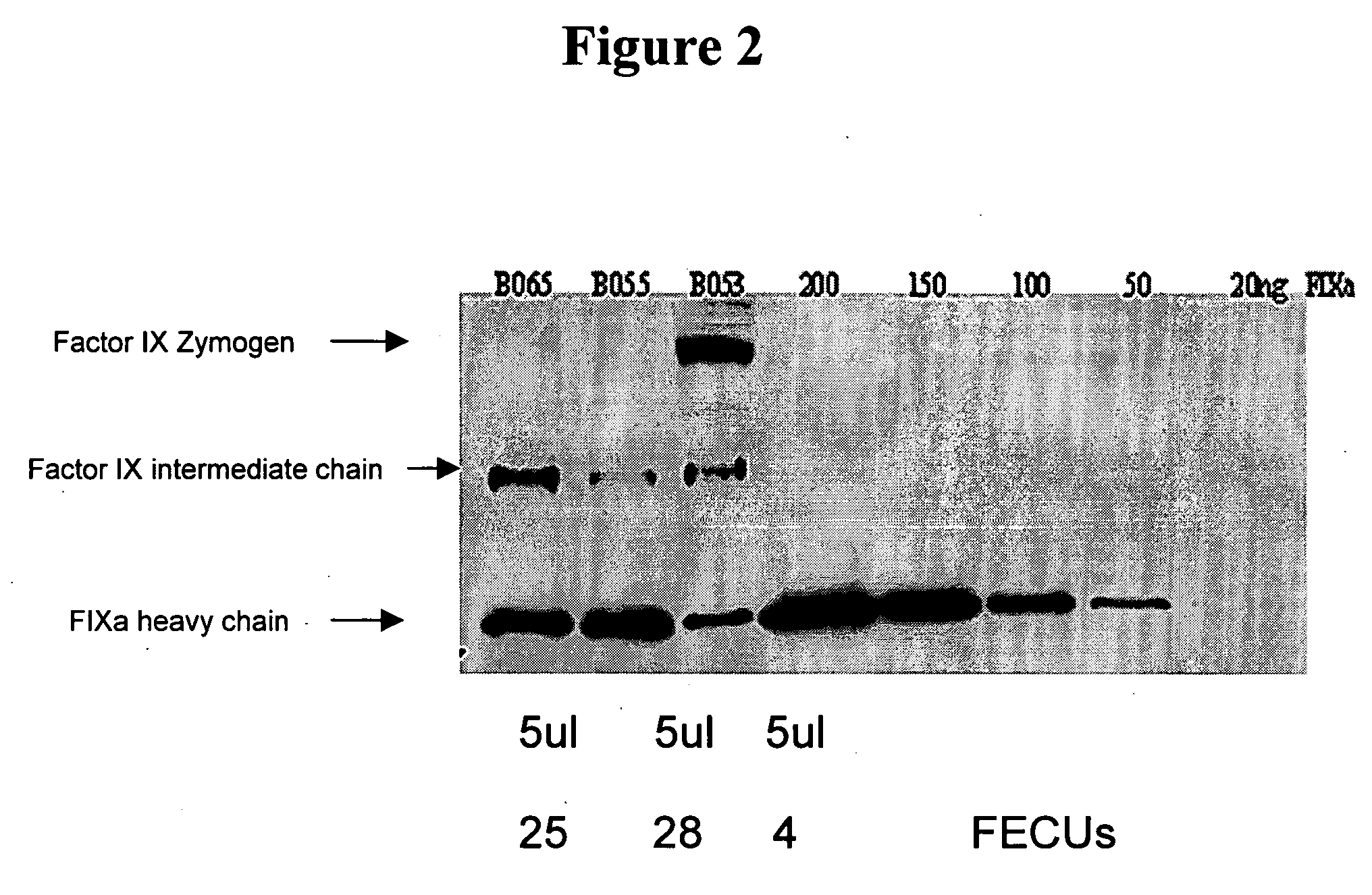
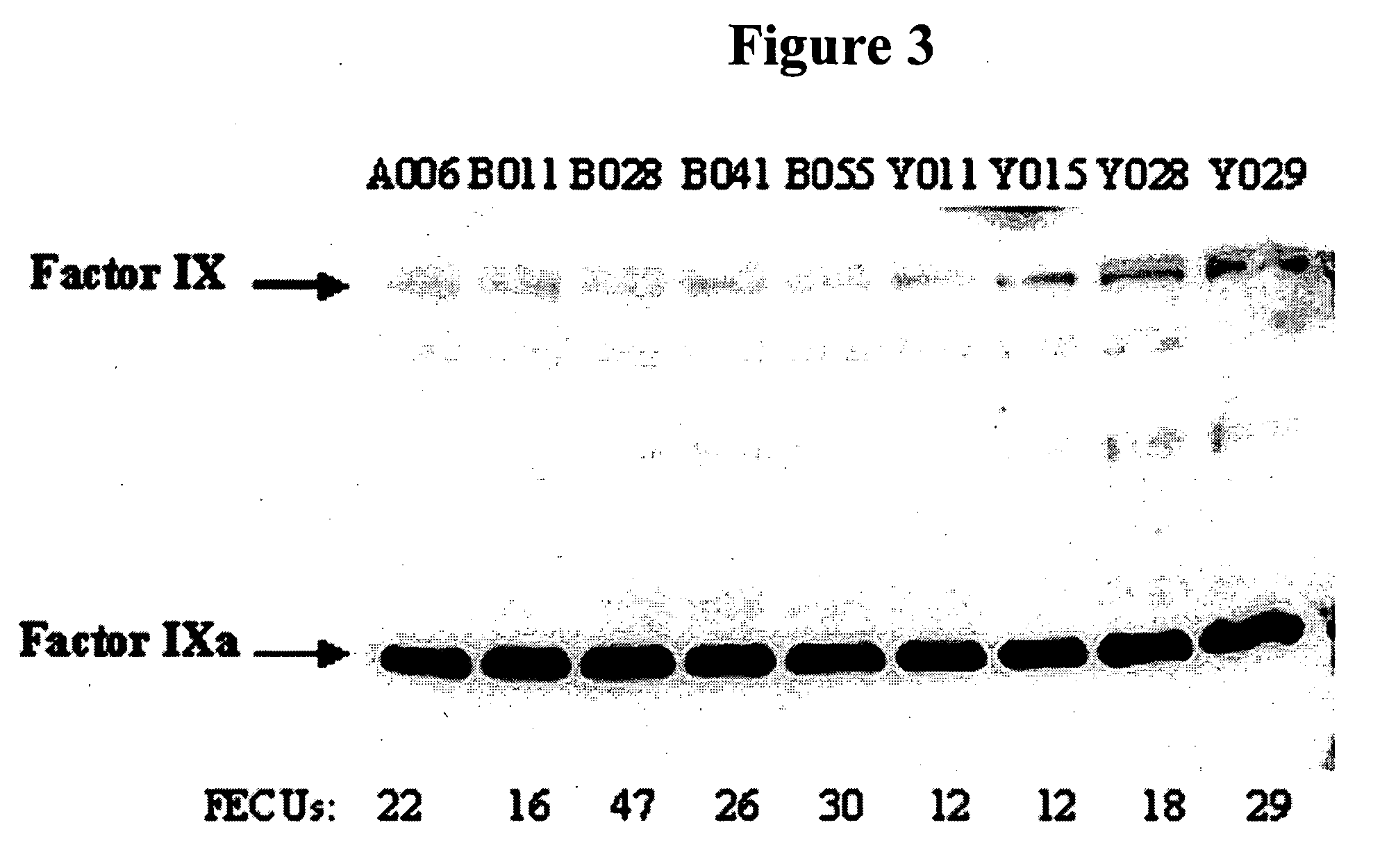
Factor IXa for the treatment of bleeding disorders
InactiveUS20050209149A1Clinical utilityCorrect the Factor VIII bleeding phenotypeFactor VIIPeptide/protein ingredientsSide effectPrekallikrein activity
The invention provides a method of treating bleeding disorders in a subject by administration of a preparation enriched for Factor IXa. The Factor IXa can be produced by proteolytically activating recombinantly-produced Factor IX. The invention also provides an improved method for producing Factor IXa from a plasma fraction, which method results in a Factor IXa product containing little or no prekallikrein activity, thus reducing the incidence of undesired side effects in a subject.
Owner:BAXALTA GMBH
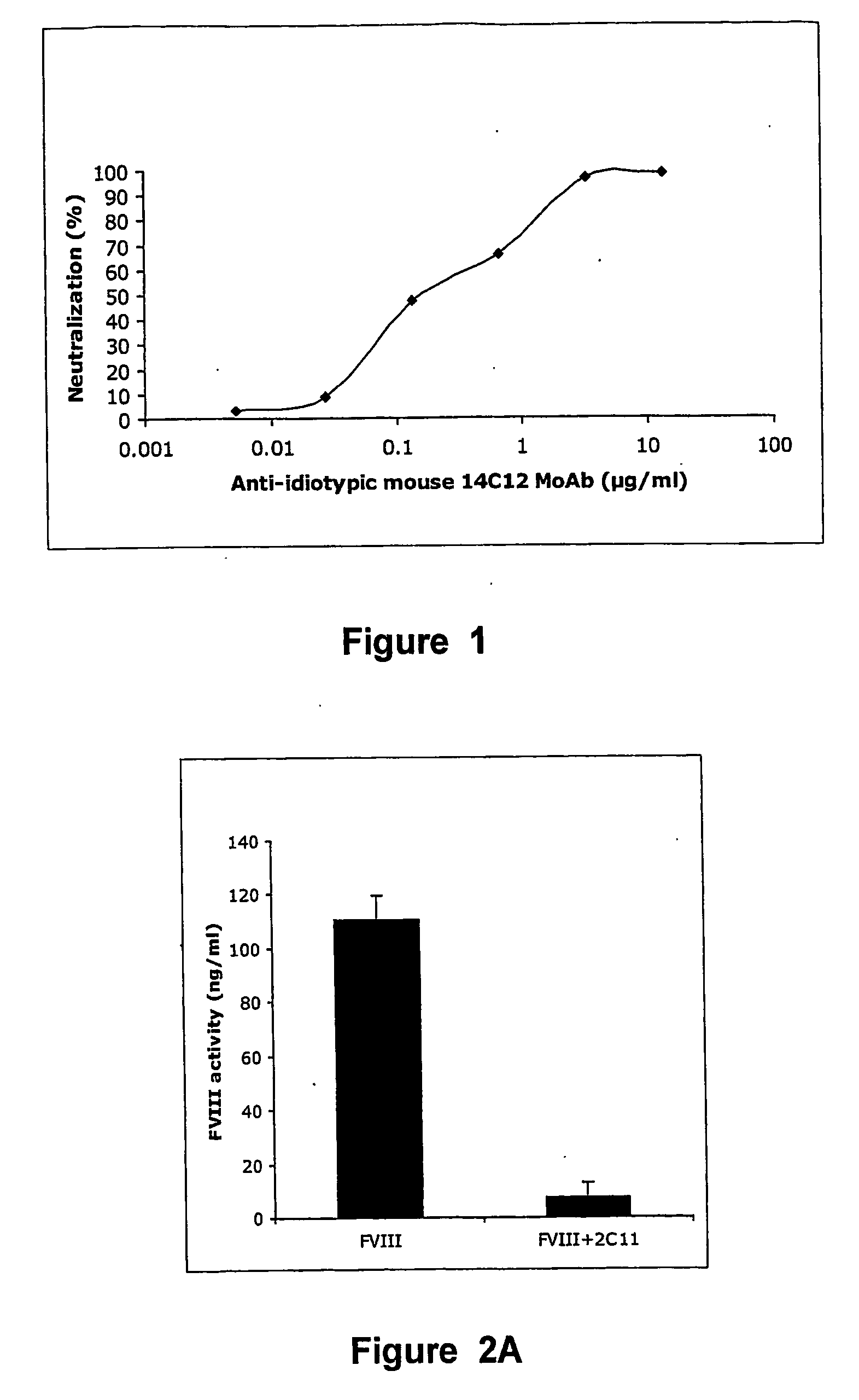
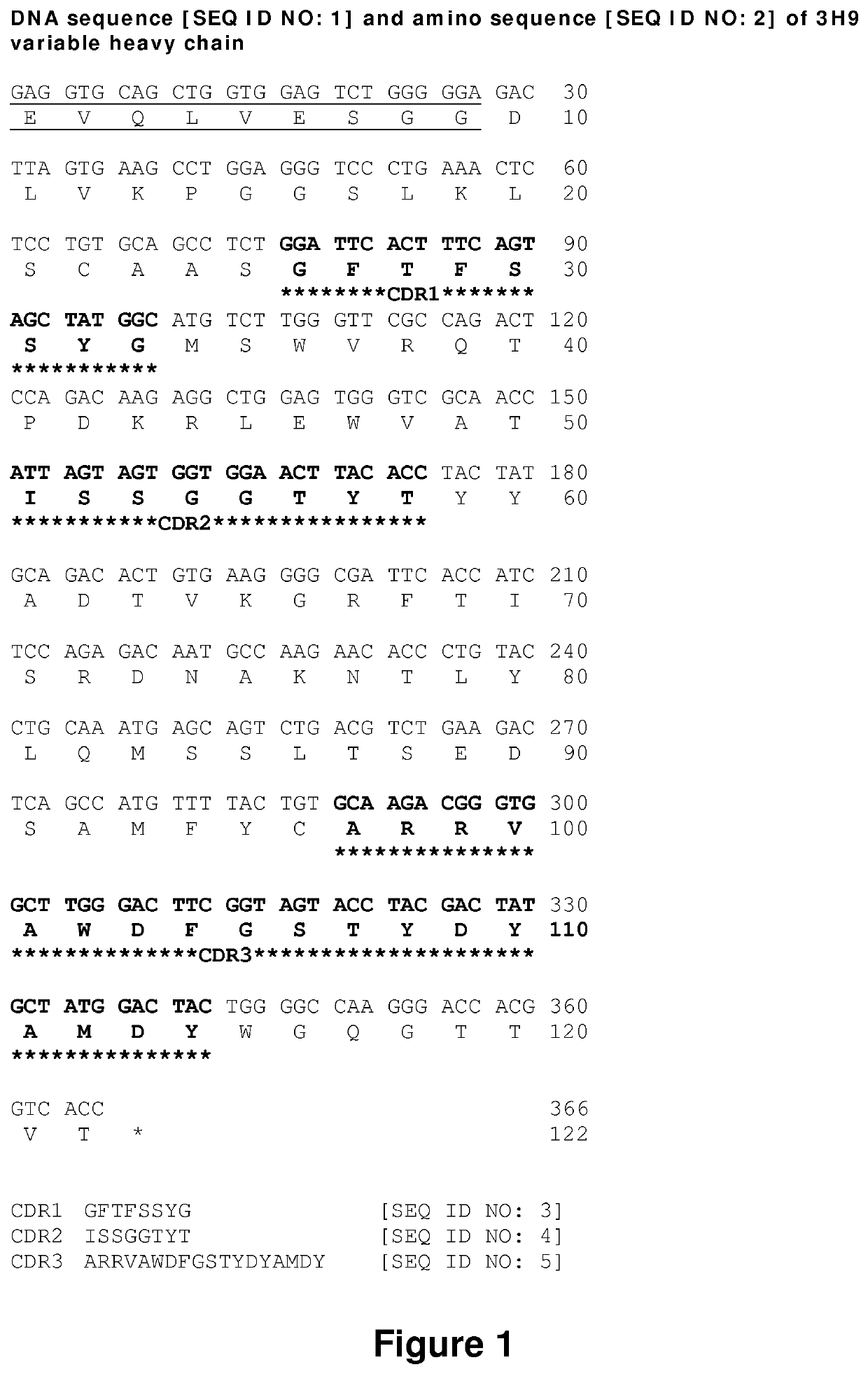
Anti-idiotypic antibodies against factor VIII inhibitor and uses thereof
InactiveUS20060239998A1Increase stability and lifetimeEnhanced inhibitory effectAnimal cellsCell receptors/surface-antigens/surface-determinantsFactor VIII inhibitorIn vivo
The present invention discloses anti-idiotypic antibodies and fragments thereof against inhibitory Factor VIII anti-bodies, said inhibitory antibodies having an affinity for the C2 domain of Factor VIII. The anti-idiotypic antibodies of the present invention are able to completely neutralise in vitro and in an in vivo mouse model the inhibitory activity of FVIII inhibitors. The anti idiotypic antibodies of the present invention can be applied for the prevention, treatment or reduction of bleeding disorders of hemophilia patients with inhibitory antibody against the C2 domain of Factor VIII.
Owner:LIFE SCI RES PARTNERS VZW
Use of VWF stabilized FVIII preparations and of VWF preparations without FVIII for extravascular administration in the therapy and prophylactic treatment of bleeding disorders
The present invention relates to the use of von Willebrand Factor (VWF) preparations or of a VWF preparation in combination with coagulation Factor VIII (FVIII) for extravascular administration in the therapy and prophylactic treatment of bleeding disorders.
Owner:CSL BEHRING GMBH
Factor VII Conjugates
InactiveUS20150225711A1Extended half-lifeImprove propertiesHydrolasesPeptide/protein ingredientsFactor VIIPolymer
The present invention relates to the conjugation of Factor VII polypeptides with heparosan polymers. The resultant conjugates may be used to deliver Factor VII, for example in the treatment or prevention of bleeding disorder
Owner:NOVO NORDISK AS
High-activity blood coagulation factor XI mutant Ala570Thr
ActiveCN112126636AImprove catalytic abilityHigh catalytic activityPeptide/protein ingredientsFermentationZymogenDisease
The present invention relates to a high-activity blood coagulation factor XI mutant Ala570Thr (A570T), nucleotide sequences are shown as SEQ ID NO: 1-4 and an amino acid sequence is shown as SEQ ID NO: 5. A zymogen state is activated into an enzyme with activity to produce resistance of a physiological inhibitor, thus the high-activity blood coagulation factor XI mutant Ala570Thr has very high coagulation activity and stronger catalytic capability on a non-physiological substrate, is applied to treatments of hemorrhagic diseases, and has very good prospects for gene treatment, gene editing andrecombinant protein replacement.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE +3
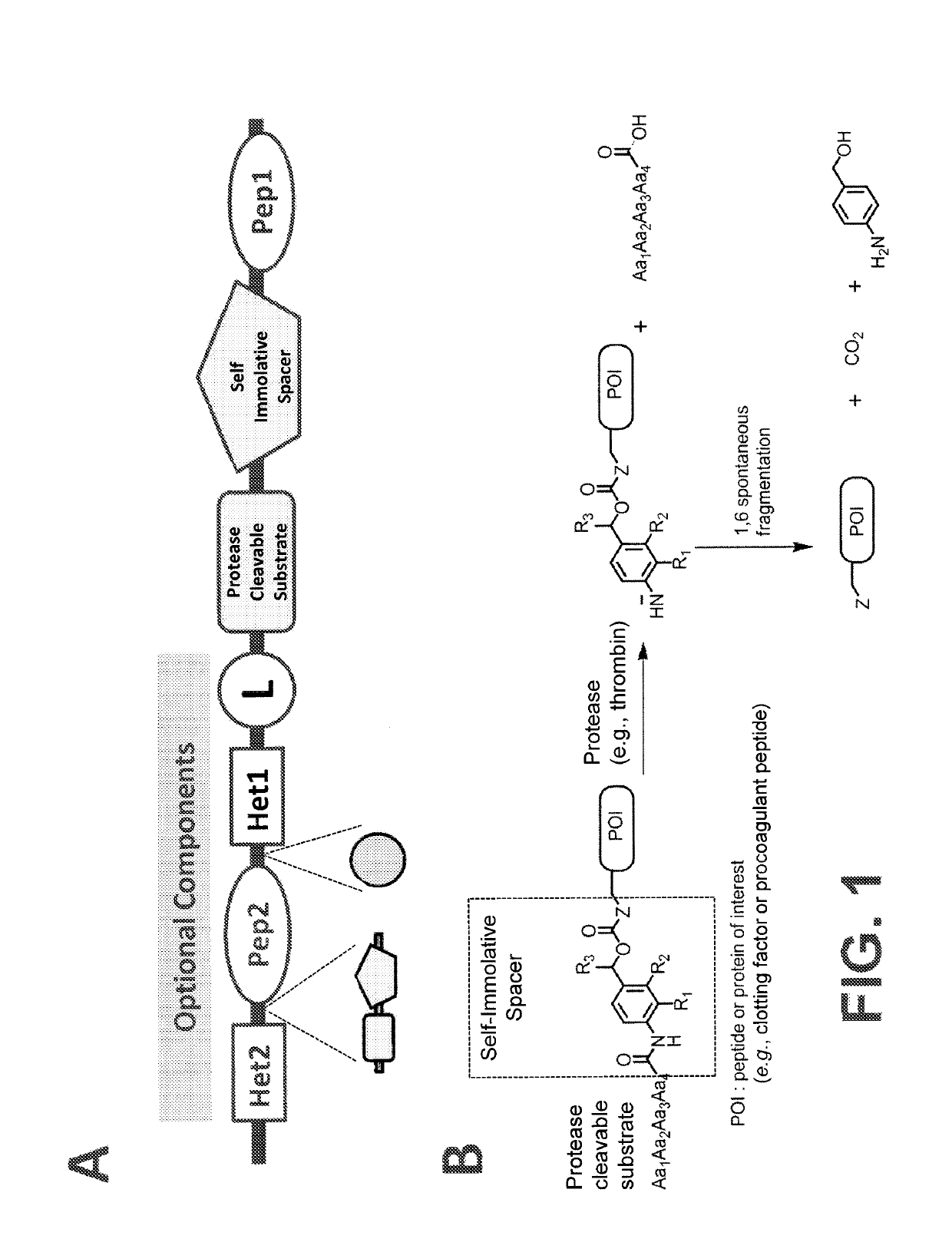
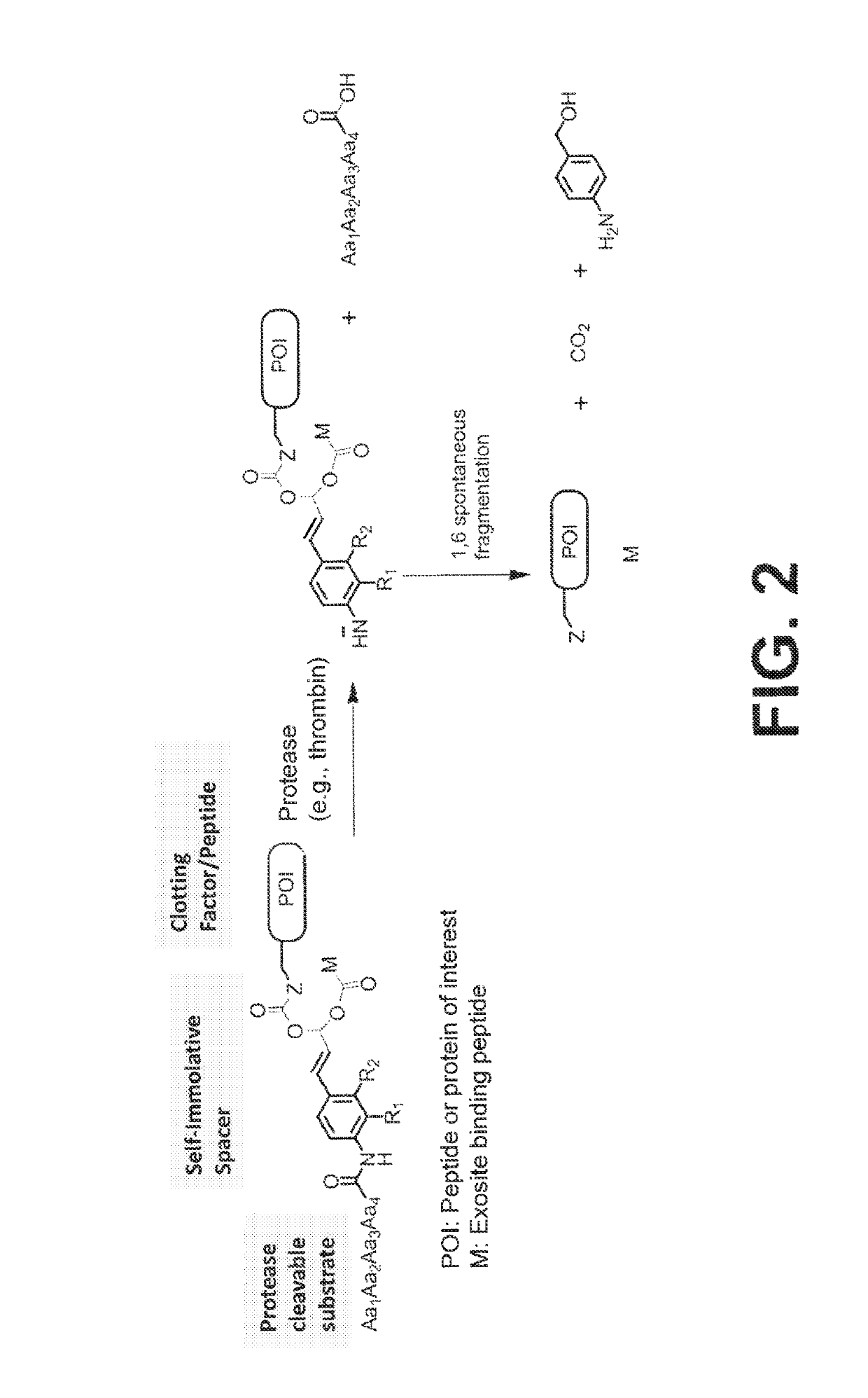
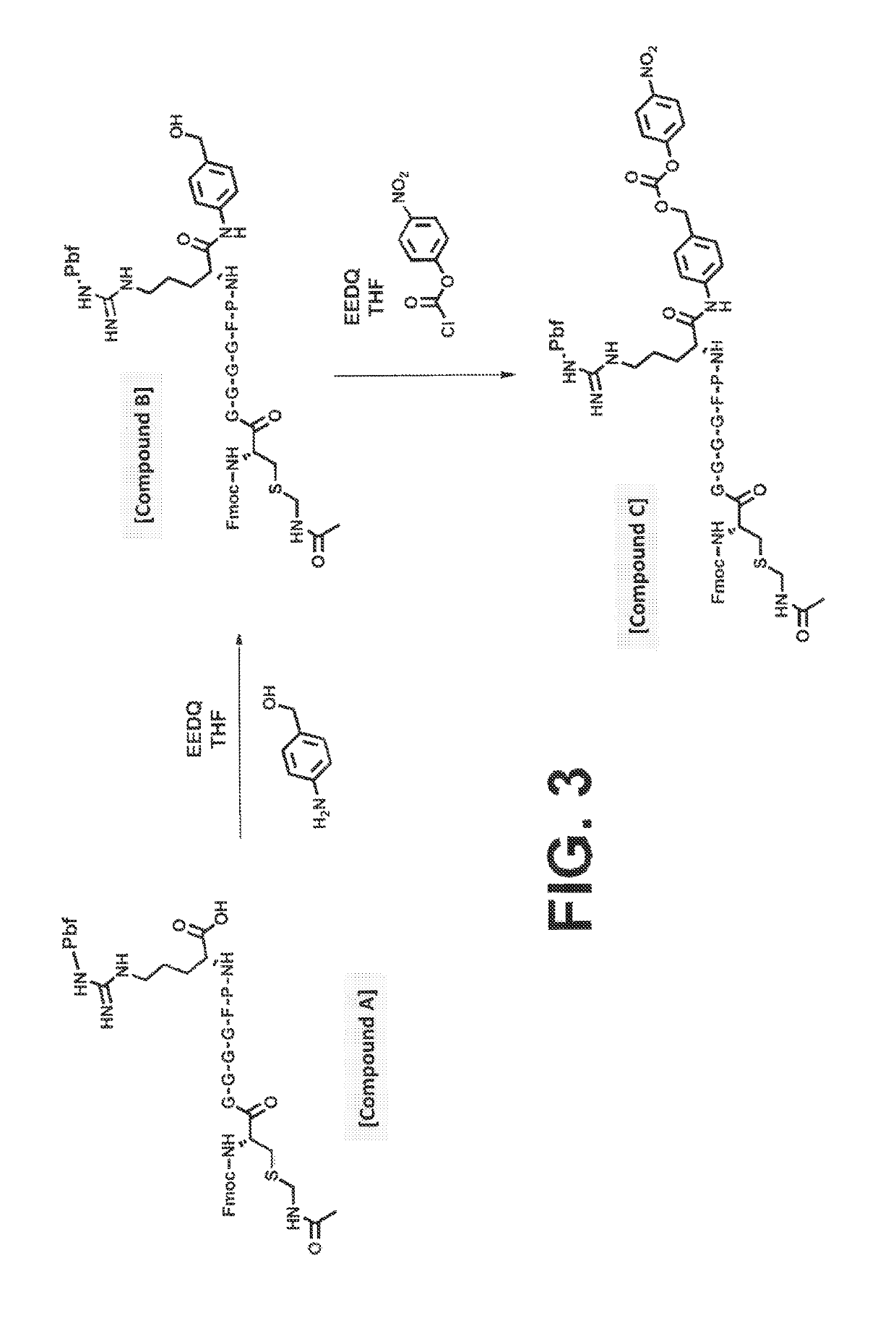
Procoagulant compounds
ActiveUS10287564B2Facilitate conjugationGood curative effectPeptide/protein ingredientsHydrolasesCarbamateThrombin activity
The present disclosure provides protease-activatable procoagulant compounds comprising a procoagulant polypeptide, e.g., a procoagulant peptide and / or clotting factor, and a linker comprising a protease-cleavable substrate (e.g., a synthetic thrombin substrate) and a self-immolative spacer (e.g., p-amino benzyl carbamate). Upon cleavage of the protease-cleavable substrate by a protease (e.g., thrombin), the self-immolative spacer cleaves itself from the procoagulant polypeptide such that the polypeptide is in an underivatized and active form. Also provided are pharmaceutical compositions, methods for treating bleeding disorders using the disclosed compounds, methods of enhancing in vivo efficacy of procoagulant polypeptides, methods of increasing the efficacy of proteolytic cleavage of compounds comprising procoagulant polypeptides, methods of activating procoagulant polypeptides, and methods of releasing a procoagulant polypeptide from a heterologous moiety such as PEG.
Owner:BIOVERATIV THERAPEUTICS INC
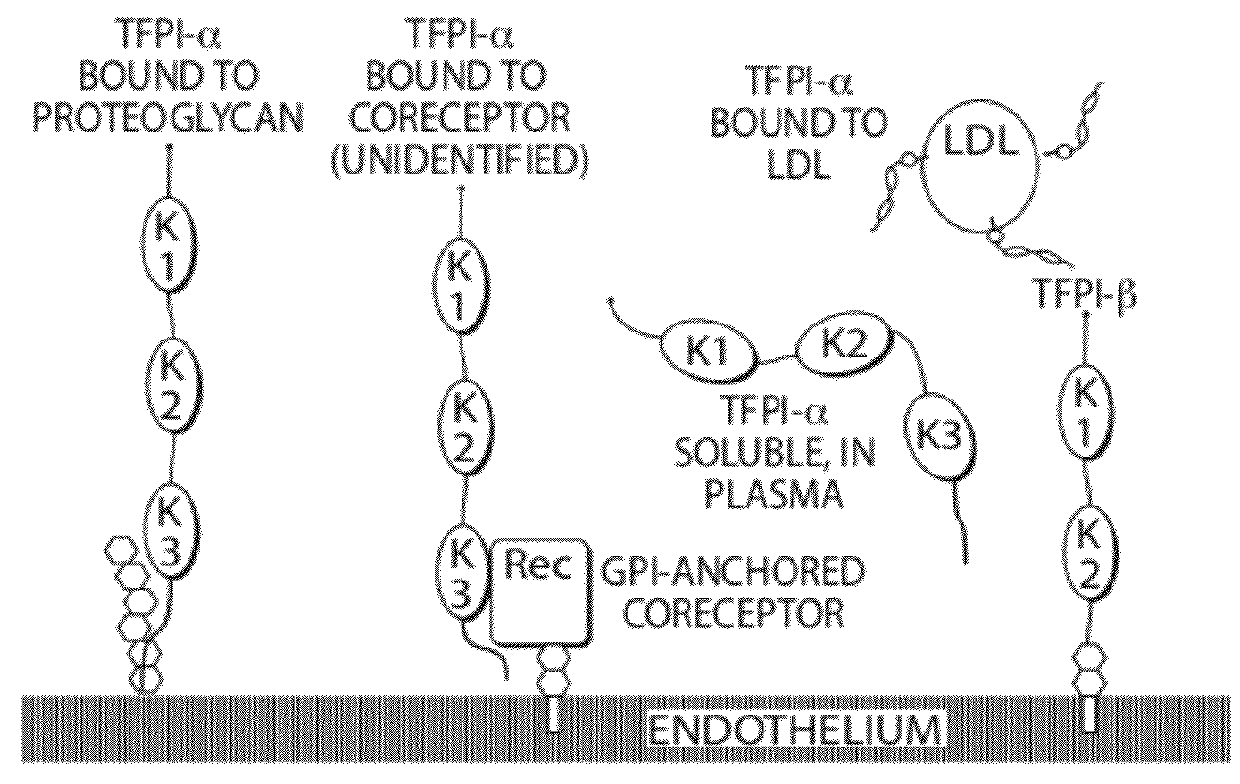
Aptamers to tissue factor pathway inhibitor and their use as bleeding disorder therapeutics
ActiveUS8252913B2Reduce complicationsEliminate the effects ofOrganic active ingredientsSugar derivativesAptamerDisease
Owner:TAKEDA PHARMA CO LTD
Methods for treating bleeding disorders
InactiveUS20140271625A1Less adenosineIncreasing platelet activationPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAdenosineBiological activation
Disclosed are methods for treating bleeding disorders, such as hemophilia, in subjects in need thereof by administering an antibody that specifically binds CD73. The methods reduce production of adenosine, increase platelet activation and / or enhance the level of coagulation on the platelet surface to reduce and / or stop bleeding. In some embodiments, the methods can further include co-administering Factor VIII to treat the bleeding disorder.
Owner:BAYER HEALTHCARE LLC
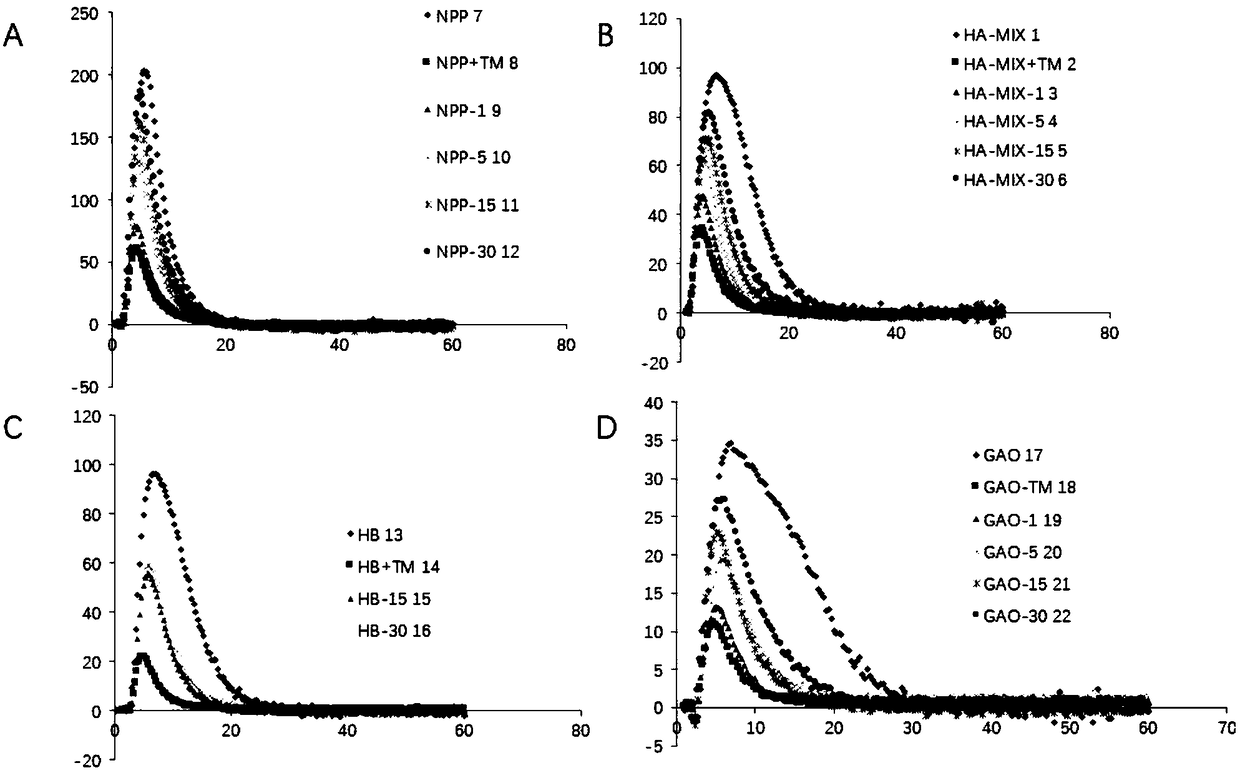
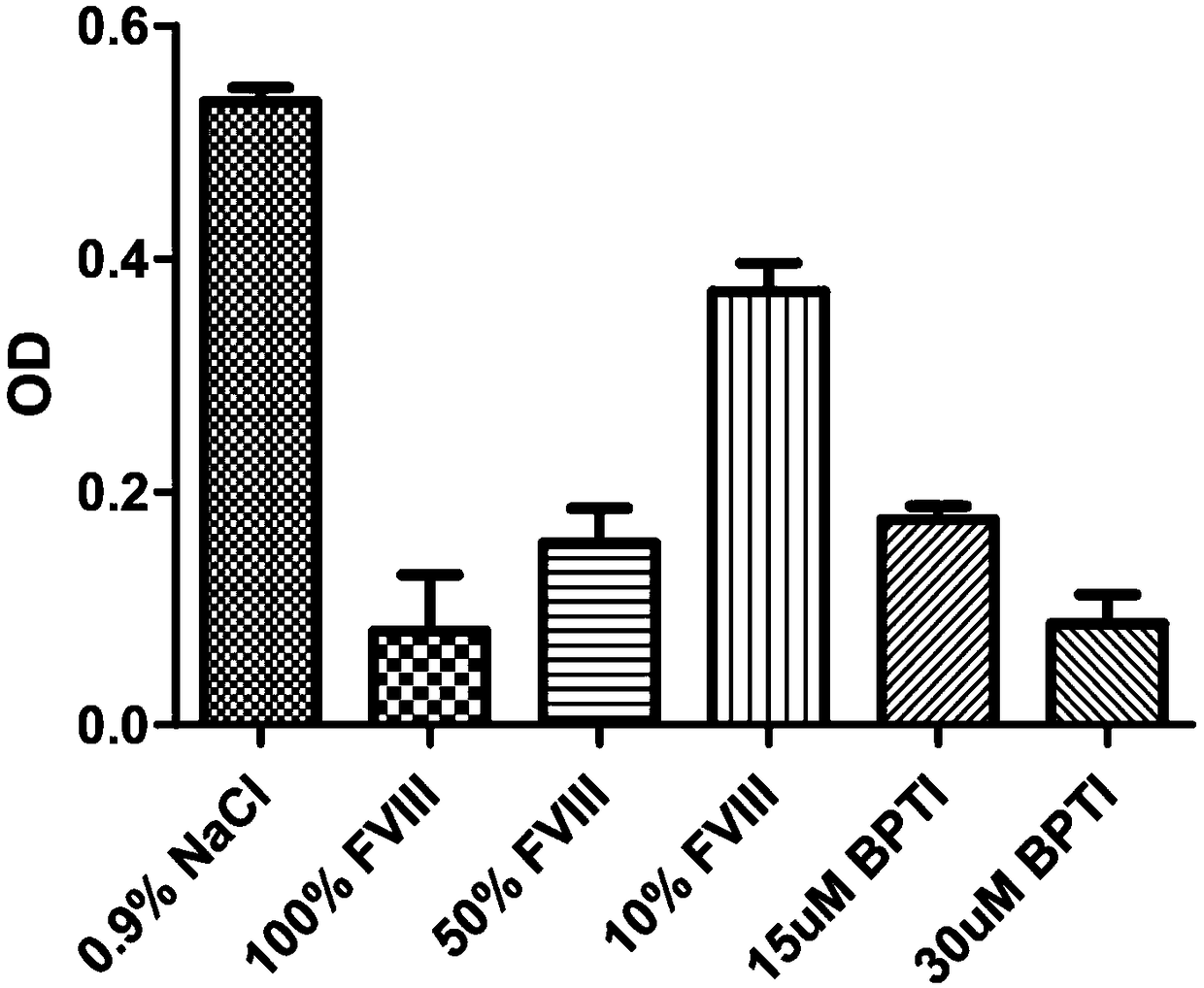
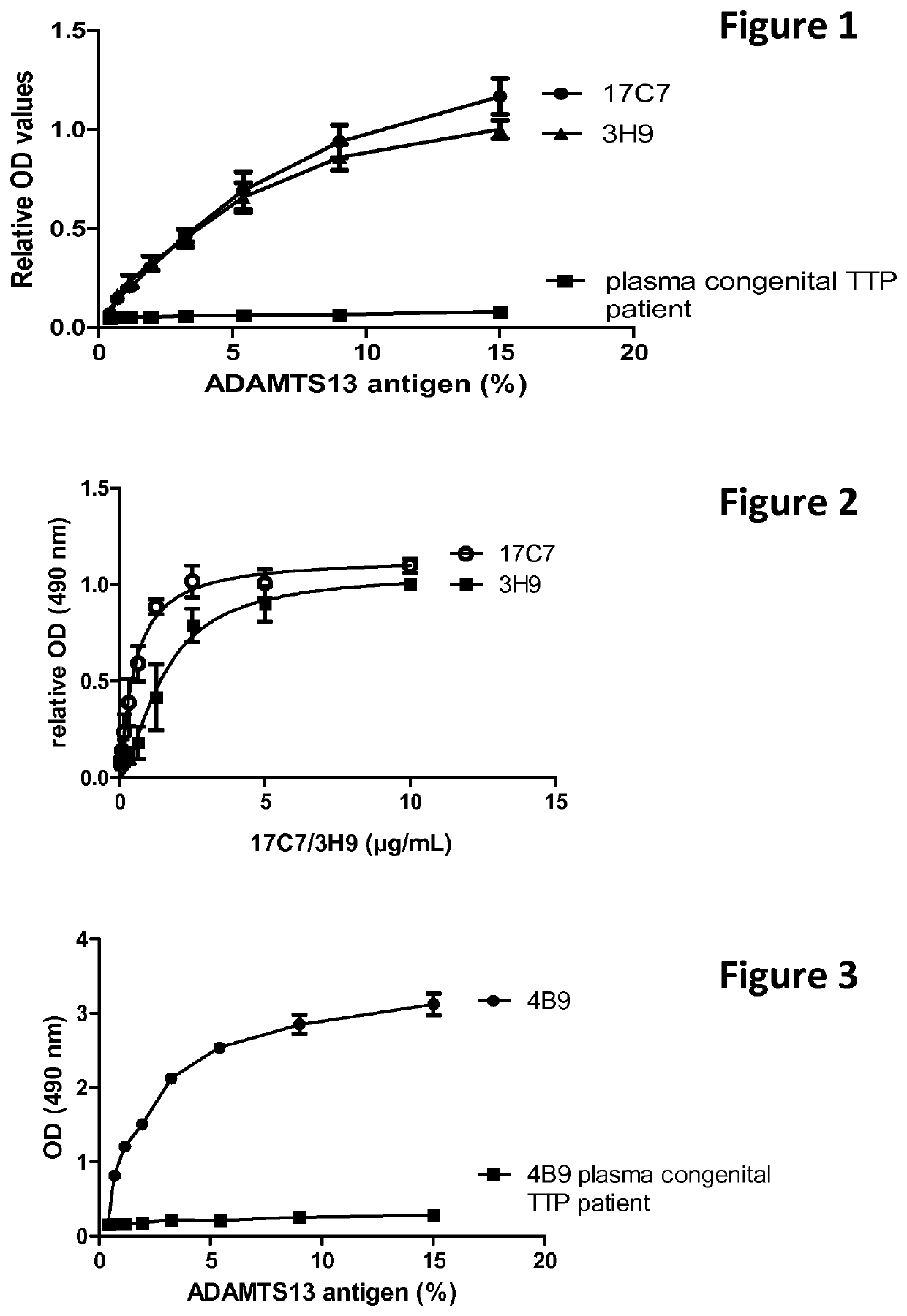
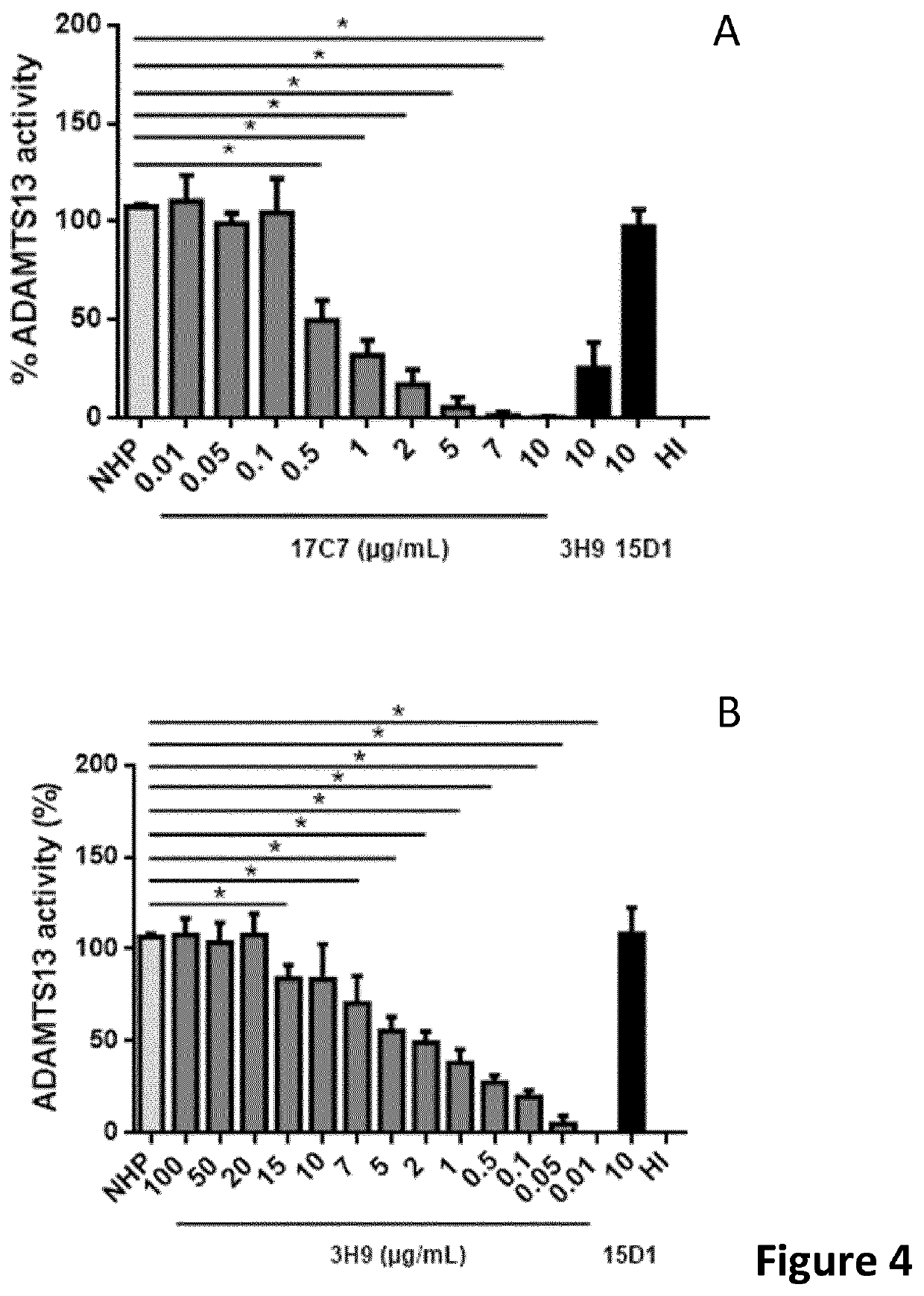
Haemorrhagic disorder due to ventricular assist device
ActiveUS10829562B2Avoid complicationsIncrease shear stressAntibody ingredientsBlood disorderDiseaseDevice implant
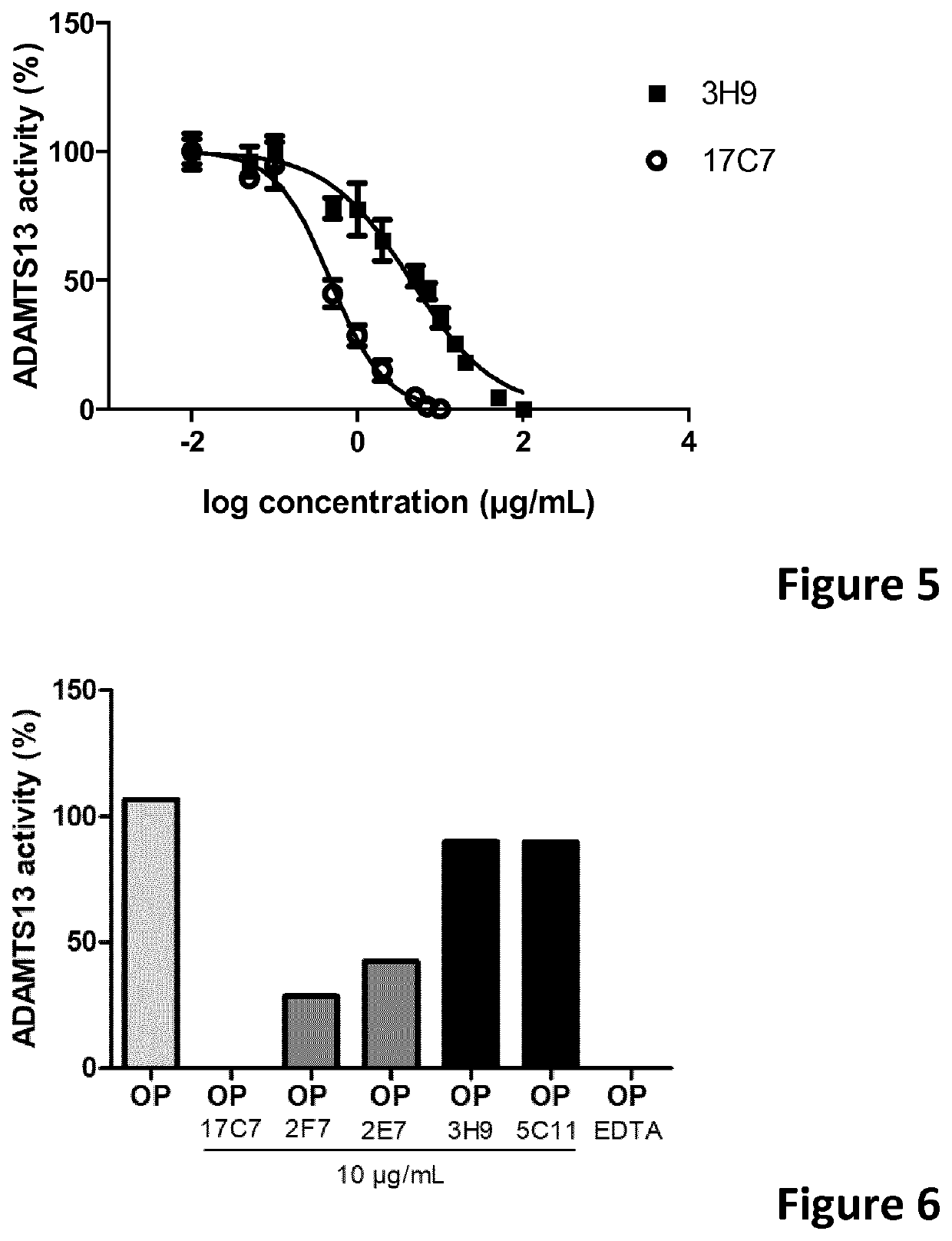
The present invention relates to methods of treatment by human ADAMTS13 inhibition in circulatory assist device induced haemorrhagic complication such as a bleeding disorder, in particular, bleeding after left ventricular assist device implantation. The present invention further relates to specific monoclonal antibodies inhibiting ADAMTS13 function.
Owner:KATHOLIEKE UNIV LEUVEN
Thrombus and hemorrhagic disease gene diagnosis method
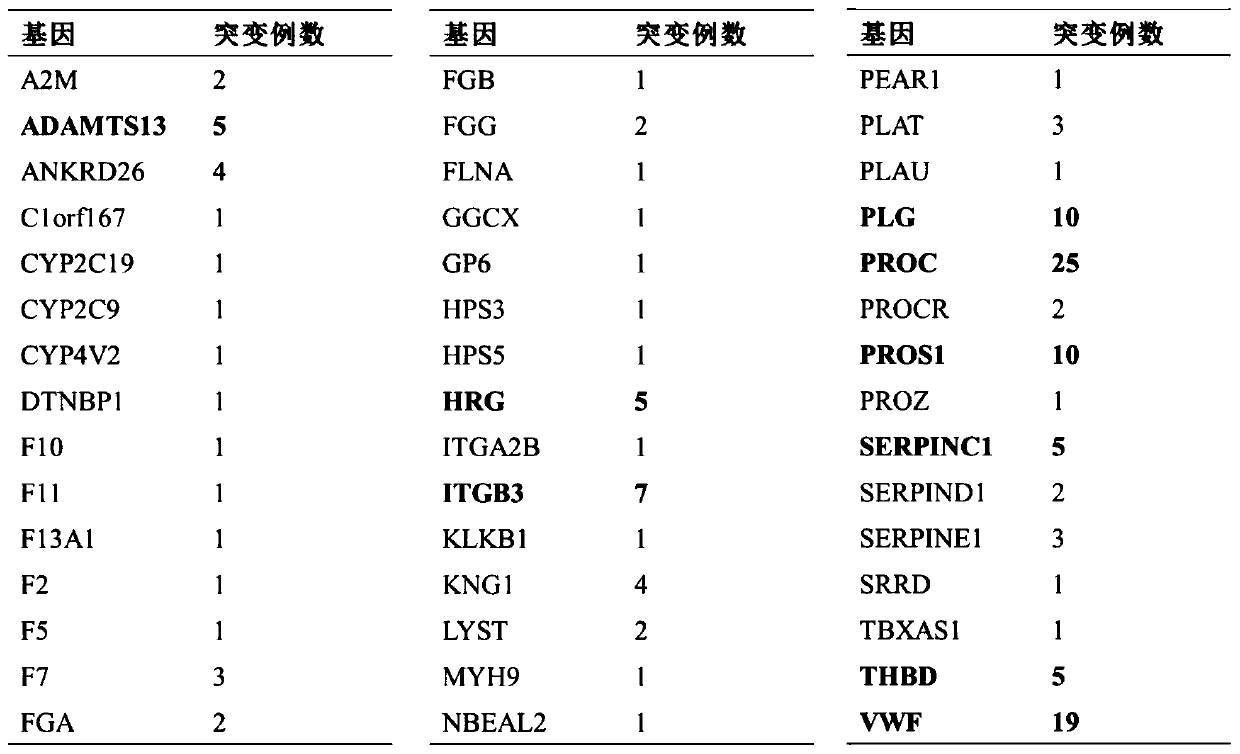
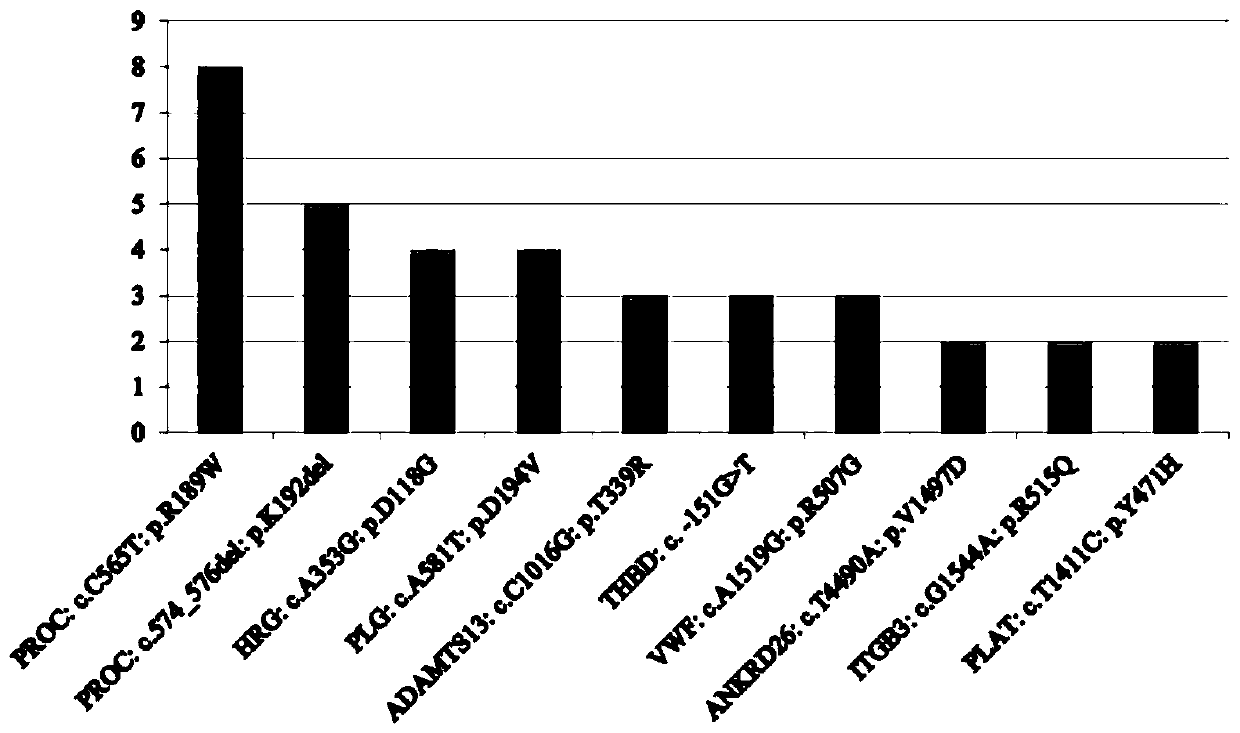
PendingCN111549128AImprove diagnosis rateIncrease coverageMicrobiological testing/measurementHuman DNA sequencingGenome human
The invention discloses a thrombus and hemorrhagic disease gene diagnosis method. All currently known 156 related genes which directly or indirectly influence blood coagulation can be comprehensivelyand systematically analyzed at a time, and key points can be placed in areas, closely related to diseases, in human genomes to find out pathogenic mutations. Most of gene variations such as point mutation, small fragment and large fragment insertion and deletion, copy number change and the like are widely screened, covered and mutated for genes involved in a blood coagulation factor system, a platelet system, a fibrinolytic system, an endothelial system, an inflammatory system, a metabolic system and an anticoagulation system at a time. Compared with other popular whole-genome sequencing technologies at present, the method is superior to whole-genome sequencing in indexes such as thrombus-related target area coverage, effective data volume, capture efficiency data utilization rate, averagesequencing depth and repetition rate, and can effectively improve the diagnosis rate of thrombotic diseases and hemorrhagic diseases.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Methods of using a fixed dose of a clotting factor
ActiveUS10391152B2Low variabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseDosing regimen
The present invention provides methods of administering a clotting factor by a fixed dosing regimen; methods of reducing, ameliorating, or preventing one or more symptoms of a bleeding disease or disorder; and a kit comprising a dotting factor useful for a fixed dosing regimen. While plasma-derived and recombinant clotting factor products allow hemophilia patients to live longer and healthier, hemophilia still remains one of the most costly and complex conditions to manage.
Owner:BIOVERATIV THERAPEUTICS INC
Methods and compositions for treating a Serpinc1-associated disorder
ActiveUS11091759B2Exceptionally effective and durable in silencing the activityExceptional potency to inhibit expression of Serpinc1Organic active ingredientsSugar derivativesDiseaseRNA - Ribonucleic acid
The invention relates to iRNA, e.g., double stranded ribonucleic acid (dsRNA), compositions targeting the Serpinc1 gene, and methods of using such iRNA, e.g., dsRNA, compositions to inhibit expression of Serpinc1 and to treat subjects having a Serpinc1-associated disease, e.g., a bleeding disorder, such as a hemophilia.
Owner:GENZYME CORP
Method for the therapeutic correction of hemophilia a by transplanting bone marrow cells
The transdifferentiation of bone marrow cells (BMCs) into hepatocytes can be used for the development of cellular medicine for degenerative and genetic diseases. Since the liver is the primary site of factor VIII (FVIII) synthesis, the partial replacement of mutated liver cells by healthy cells in hemophilia A (HA) could manage the severity of the bleeding disorder. The use of BMCs could be used as a therapy for the bleeding phenotype of hemophilia A and other related disorders.
Owner:NATIONAL INSTUTUTE OF IMMUNOLOGY
Aprotinin or application of mutant, derivative and analogue or combination fragment thereof
InactiveCN109316599AInhibition of dissolutionImprove coagulation functionPeptide/protein ingredientsBlood disorderDiseaseAprotinin
The invention relates to aprotinin or application of a mutant, a derivative and an analogue or a combination fragment thereof. The aprotinin is used for preparing a medicine for treating related diseases or symptoms with acquired hemophilia and hemophilia with production of inhibitors. The aprotinin can be used for preparing the medicines for hemorrhagic diseases, especially related medicines forhemophilia with inhibitors, and the prospect is good.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE +3
Humanised adamts13 binding antibodies
ActiveUS20200308303A1Improve propertiesAvoid complicationsAntibody ingredientsBlood disorderDiseaseDevice implant
The present invention further relates to specific humanised monoclonal antibodies inhibiting ADAMTS13 function. The present invention relates to methods of treatment by human ADAMTS13 inhibition in circulatory assist device induced haemorrhagic complication such as a bleeding disorder, in particular, bleeding after left ventricular assist device (LVAD) implantation.
Owner:KATHOLIEKE UNIV LEUVEN
Haemorrhagic disorder due to ventricular assist device
ActiveUS20180362665A1Increase in sizeIncrease the gapAntibody medical ingredientsBlood disorderMedicineLeft ventricular size
The present invention relates to methods of treatment by human ADAMTS13 inhibition in circulatory assist device induced haemorrhagic complication such as a bleeding disorder, in particular, bleeding after left ventricular assist device implantation. The present invention further relates to specific monoclonal antibodies inhibiting ADAMTS13 function.
Owner:KATHOLIEKE UNIV LEUVEN
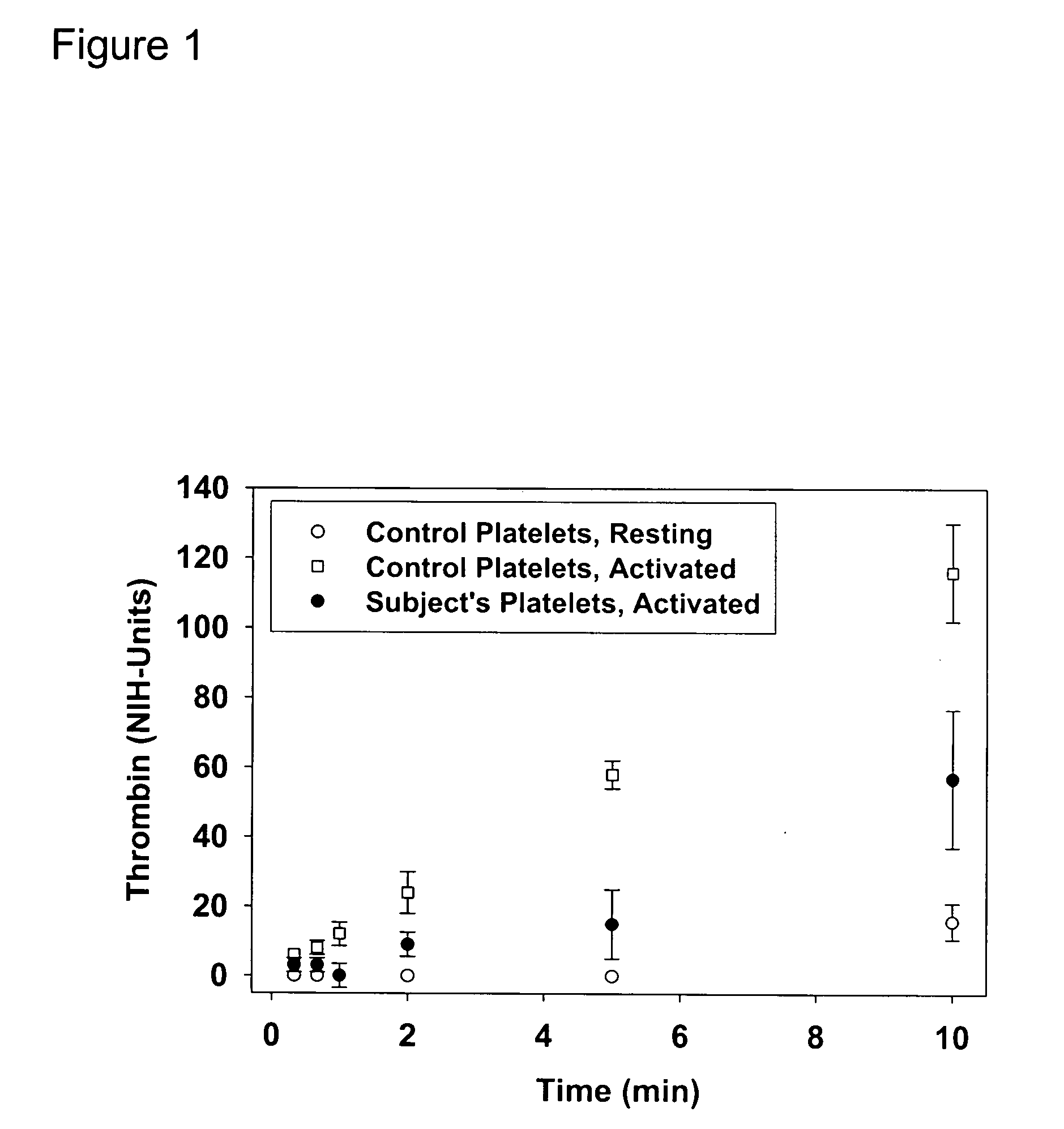
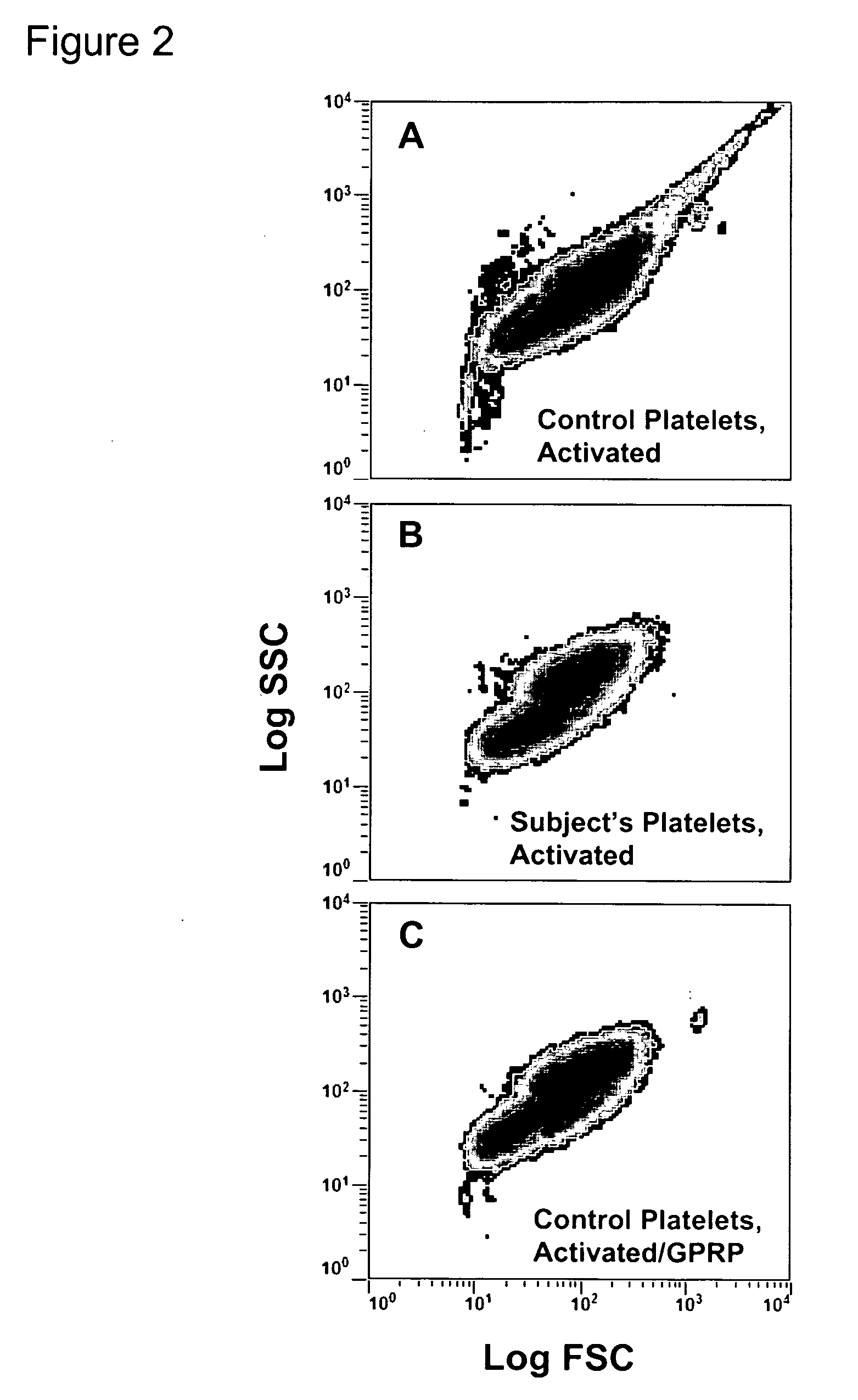
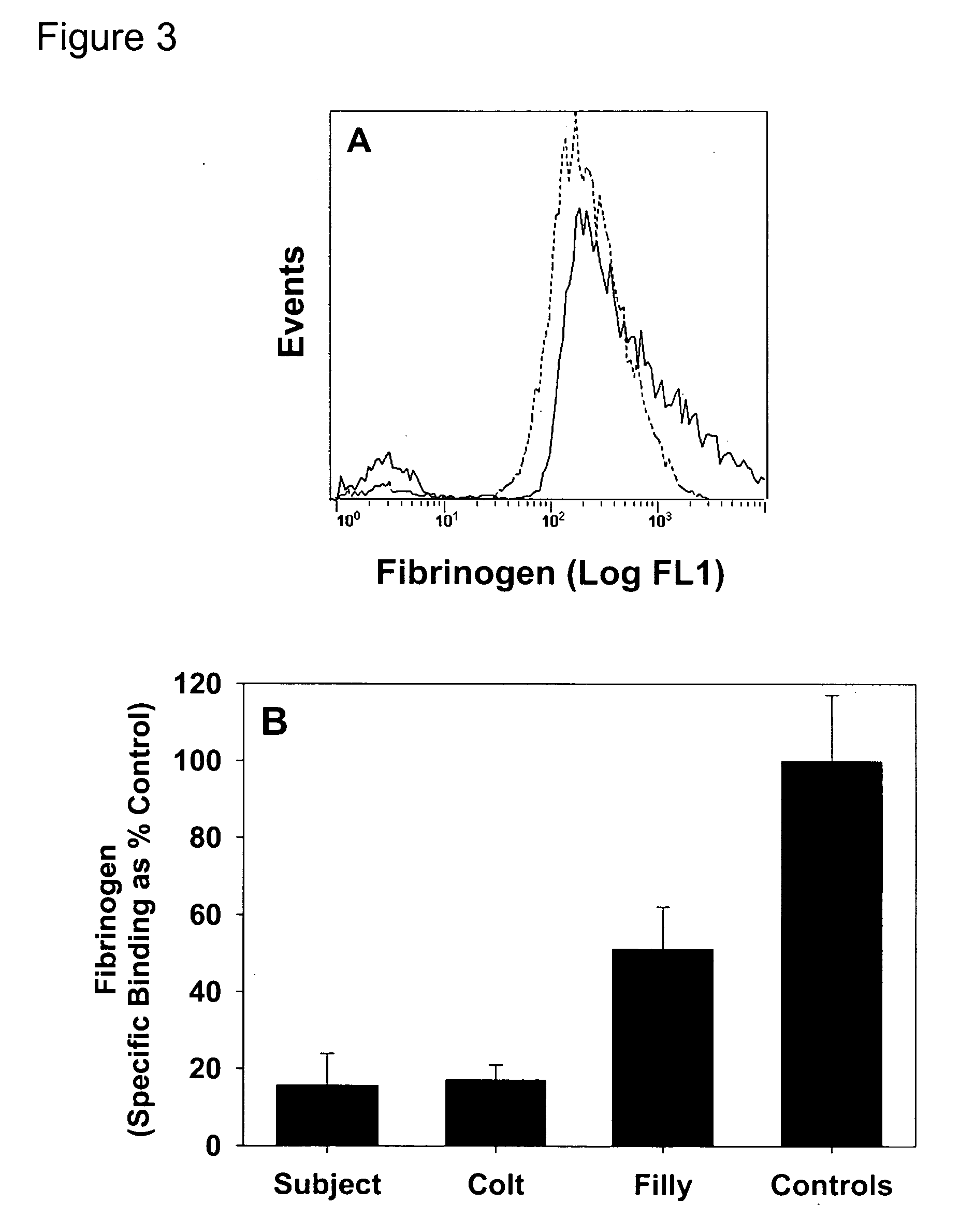
Thrombin-induced fibrinogen binding for the detection of risk of bleeding disorders
InactiveUS20070059777A1Reduce decreaseIncreased riskBiological testingThrombin activityFibrinogen binding
This invention provides a methods of detecting a bleeding disorder in mammals where the bleeding disorder is characterized by normal fibrinogen binding to ADP-activated platelets, but decreased fibrinogen binding to thrombin-activates platelets.
Owner:RGT UNIV OF CALIFORNIA
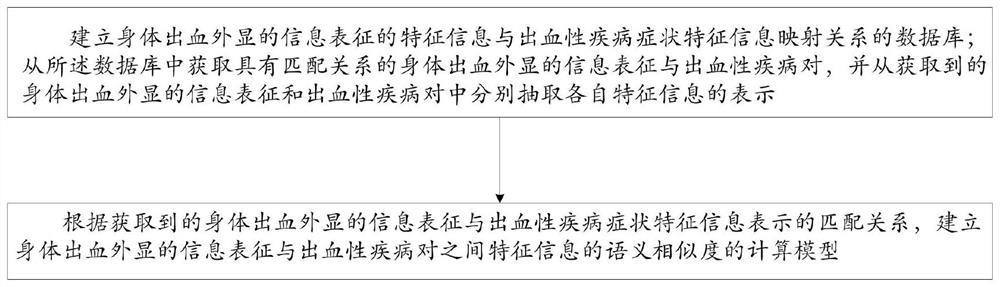
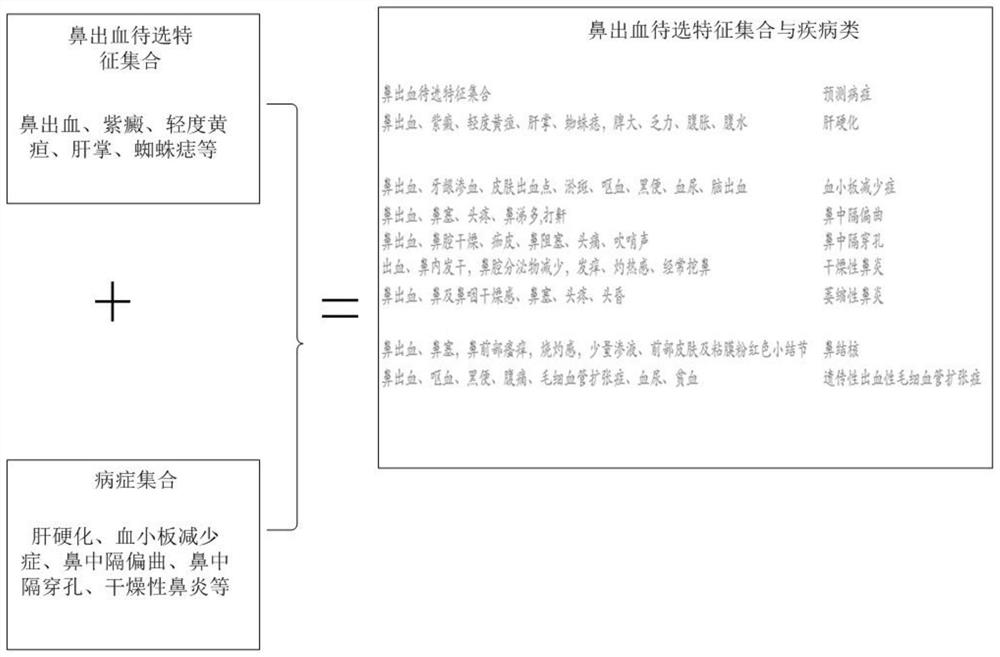
Intelligent prediction model construction method for hemorrhagic diseases, and prediction system
The invention provides an intelligent prediction model construction method for hemorrhagic diseases. The method comprises the following steps: establishing a database of a mapping relationship betweenfeature information of body hemorrhage explicit information representation and hemorrhagic disease symptom feature information; and establishing a calculation model of the semantic similarity of thefeature information between the body hemorrhage explicit information representation and the hemorrhagic disease pair according to the obtained matching relationship between the body hemorrhage explicit information representation and the hemorrhagic disease symptom feature information representation. According to the method, semantic cognition of the relationship between the feature information ofthe body hemorrhage explicit information representation and the disease is realized through the construction of the intelligent prediction model of the hemorrhagic disease, so that the category of thehemorrhagic disease, the bleeding reason and the possible disease are intelligently predicted or evaluated and diagnosed in an assisted manner.
Owner:吾征智能技术(北京)有限公司
Internal traditional Chinese medicine composition for treating a hemorrhagic disease of fish and preparation method of internal traditional Chinese medicine composition
InactiveCN104666506AImprove the environmentGuaranteed survivalBlood disorderPlant ingredientsDracocephalumIntestinal bleeding
The invention relates to an internal traditional Chinese medicine composition for treating a hemorrhagic disease of fish and a preparation method of the internal traditional Chinese medicine composition. The internal traditional Chinese medicine composition comprises the following components in parts by weight: 10-20 parts of rhizoma polygoni paleacei, 10-20 parts of gardenia, 10-20 parts of dracocephalum moldavuca linn, 5-20 parts of rhizoma dryopteris crassirhizomae and 10-30 parts of diversecolor cinquefoil. The traditional Chinese medicine composition provided by the invention is scientific in compatibility, simple in process, convenient to use, relatively low in cost, and free of a medicine residue and a toxic or side effect, and can effectively play good roles in prevention and treatment of red muscle type, red-fin and red-operculum type and enteritis intestinal bleeding.
Owner:TIANJIN REBATE SCI & TECH DEV
Activated partial thromboplastin generation combined correction test and detection kit
PendingCN114200144AProlongation of thromboplastin timeDisease diagnosisBiological testingDiseaseMedicine
The invention relates to a method for simultaneously detecting five hemorrhagic diseases clinically and a preparation method of a kit. The method is characterized by comprising the following steps: (1) preparing a kit containing fresh mixed plasma, barium sulfate adsorbed plasma and stored serum; and (2) adding a certain amount of plasma of a patient to be detected into each tube of the kit, and respectively detecting activated partial thromboplastin time (APTT) of each tube. The five hemorrhagic diseases are diagnosed in a laboratory according to the comparison condition (correction condition) of the APTT time of the reagent containing tube and the APTT time of the plasma of the patient. And (3) diluting the mixed plasma with different concentrations by using the stored serum and the barium sulfate adsorbed plasma respectively, determining activated partial thromboplastin time (APTT) to obtain standard curves of factors VIII and IX, and quantitatively determining the factors VIII and IX of the patient.
Owner:周丽艳
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com