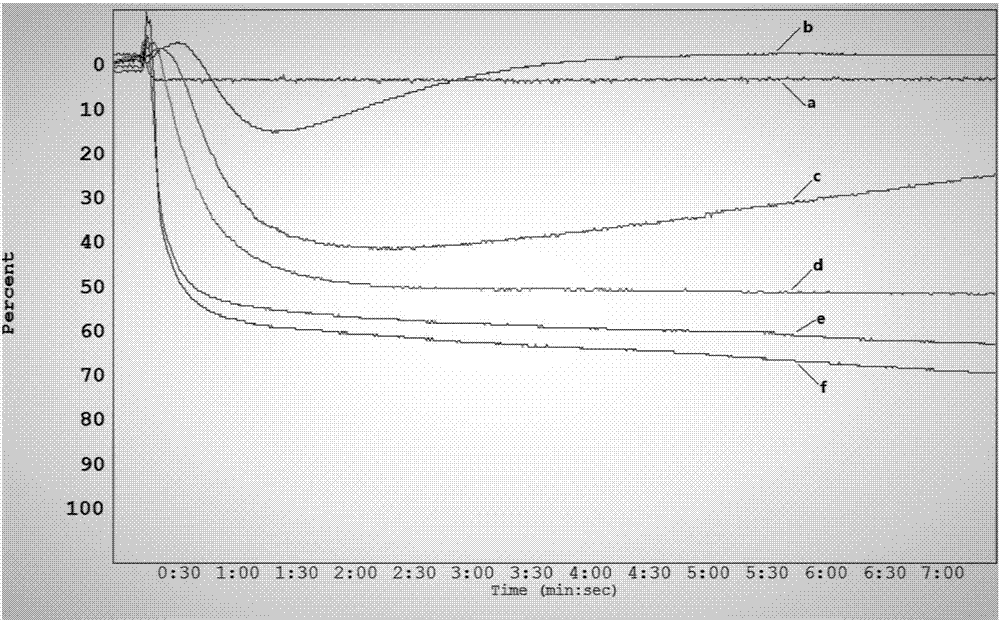
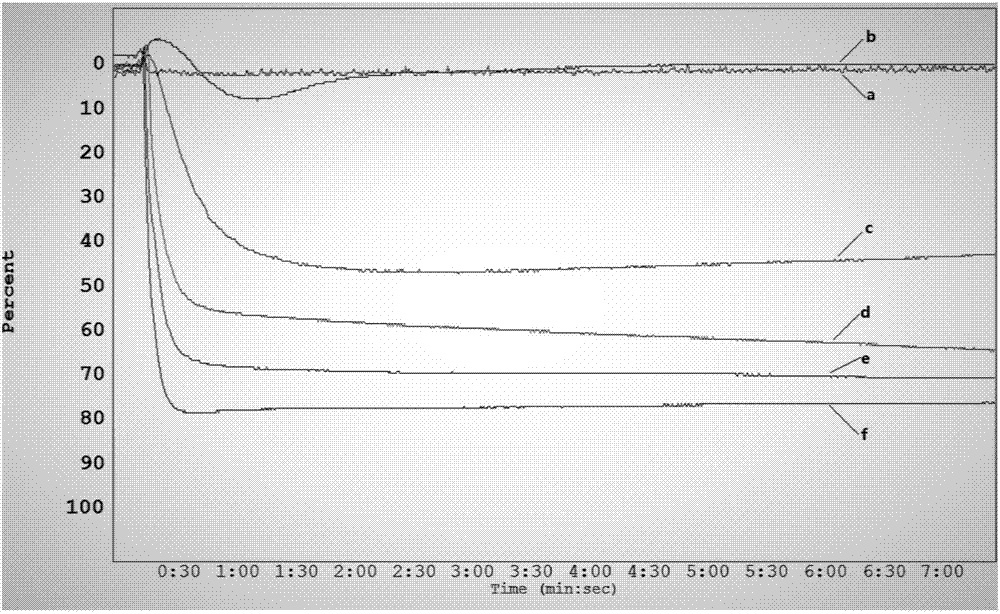
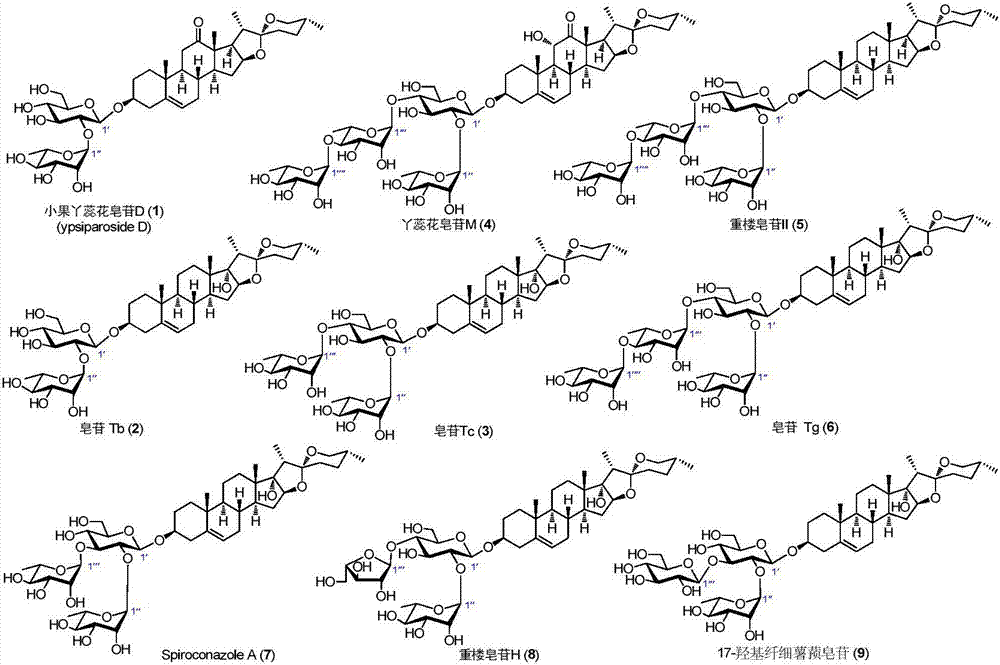
C27 spirostane-type steroidal saponin compound and pharmaceutical composition and application thereof
A technology of steroid saponins and spirostane, which is used in C27 spirostane type steroid saponins and their pharmaceutical compositions, applications in medicines, and application fields in the preparation of functional daily chemicals and health care products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The preparation of six compounds: saponin D, saponin M, saponin Tb, saponin Tc, saponin Tg and pachyflour saponin II:
[0047] 30kg of dried whole herb of Arthia japonica was crushed and extracted 3 times with 75% ethanol under reflux (2 hours each time), the extracts were combined, and the ethanol was recovered under reduced pressure to obtain an aqueous solution. Then the aqueous solution was passed through a macroporous resin (model: YWD-03F; dosage: 12L) column, and eluted with water, 30% EtOH, 70% EtOH, and 95% EtOH, respectively. The solvent of the 70% ethanol elution part was recovered and dried separately to obtain 1.5 kg of 70% EtOH eluate, mixed with 3 kg of silica gel (80-100 mesh), and subjected to 15 kg of silica gel (200-300 mesh) wet column chromatography, Gradient elution with chloroform-methanol-water (10:1:0→8:2:0.2→7:3:0.5), and finally washing the column with methanol, TLC detection and combined to obtain 5 components (Fr.1-Fr .5). Fr.3 (chloroform...
Embodiment 2
[0049] Preparation of saponin Tb, saponin Tc and saponin Tg three compounds:
[0050] Dried Jilin Trillium (Trilliumkamtschaticum) whole herb (10kg) was pulverized and then refluxed with 75% ethanol for 3 times for 2 hours each time. The extracts were combined and the ethanol was recovered under reduced pressure. The recovered solution was extracted three times with n-butanol, concentrated and recovered under reduced pressure to obtain 839.4 g of n-butanol extract. After the n-butanol extract was dissolved, it was eluted with water, 30% EtOH, 70% EtOH, and 95% EtOH respectively through a macroporous adsorption resin column. The solvent in the 70% EtOH eluted part was recovered to dryness respectively to obtain 170.2 g of 70% EtOH eluted product. After 70% ethanol elution was decolorized by MCI, 1700g of silica gel (300-400 mesh) was used for dry packing and loading, and gradient elution was carried out with chloroform-methanol-water (8:2:0.2,7:3:0.5) , combined to obtain 4 p...
Embodiment 3
[0052] Preparation of saponin Tb, saponin Tg, saponin II, 17-hydroxy diosgenin, Spiroconazole A and saponin H, six compounds:
[0053]5 kg of dried rhizomes of Paris polyphylla var. yunnanensis were crushed and extracted three times with 75% ethanol under reflux (2 hours each time). The extracts were combined, and the ethanol was recovered under reduced pressure to obtain an aqueous solution. Then the aqueous solution was passed through a macroporous resin (model: D-101) column, and eluted with water, 30% EtOH, 70% EtOH, and 95% EtOH, respectively. The solvent of the 70% elution part was recovered and dried separately to obtain 225g of 70% EtOH eluate, which was mixed with 450g silica gel (80-100 mesh) and subjected to 2200g silica gel (200-300 mesh) wet column chromatography. ‐methanol‐water (10:1:0→8:2:0.2→7:3:0.5) gradient elution, and finally wash the column with methanol, TLC detection combined to obtain 4 components (Fr.1‐Fr.5 ). Part of Fr.3 (chloroform-methanol-water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com