High-activity blood coagulation factor XI mutant Ala570Thr
A technology of coagulation factor and high activity, applied in blood diseases, genetic engineering, plant genetic improvement, etc., can solve problems such as low efficiency and limitations, achieve strong catalytic ability, improve overall coagulation function, and high coagulation activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
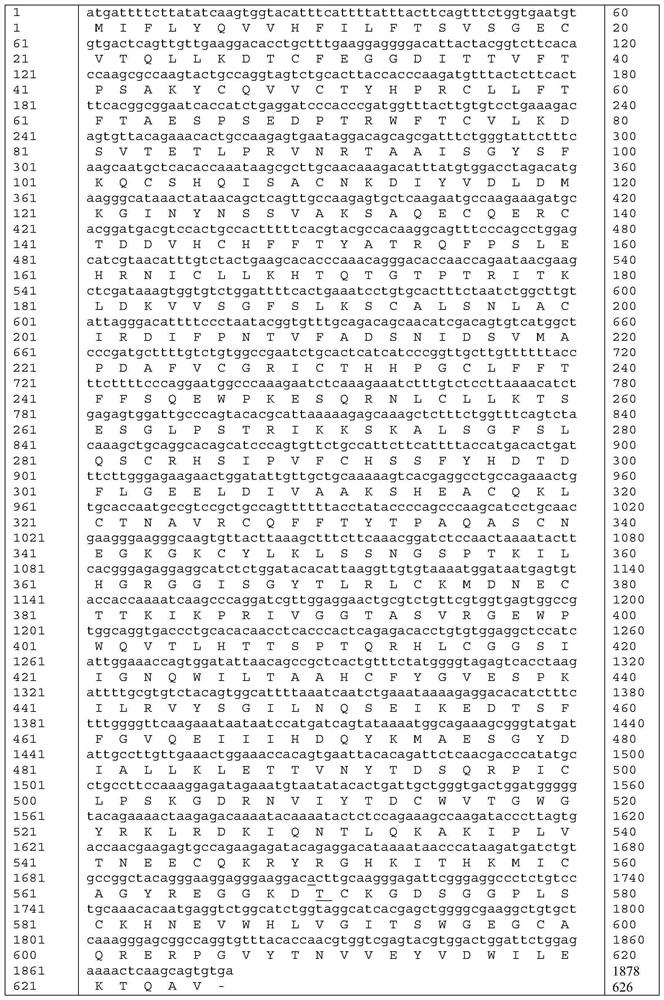
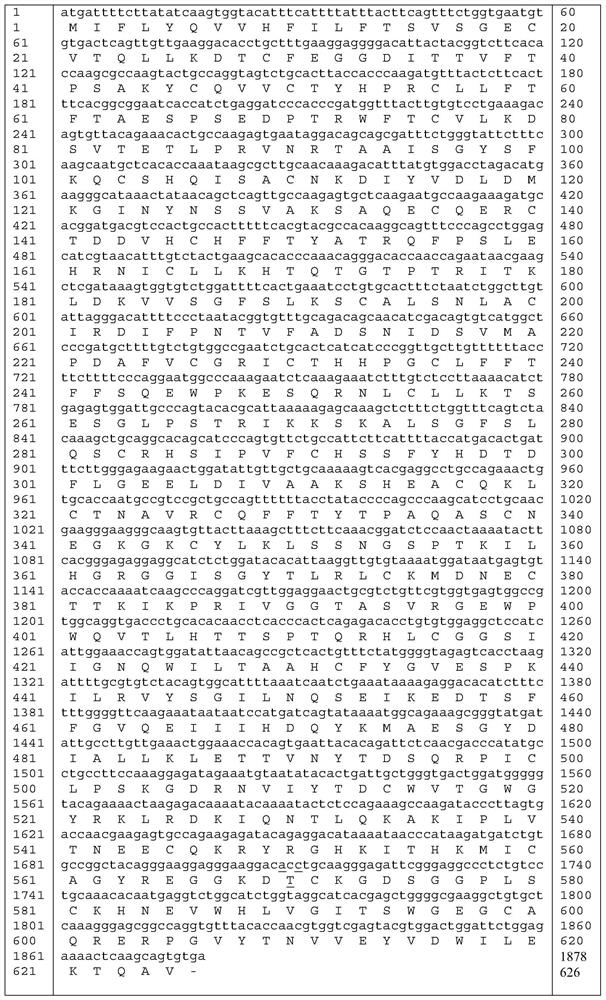
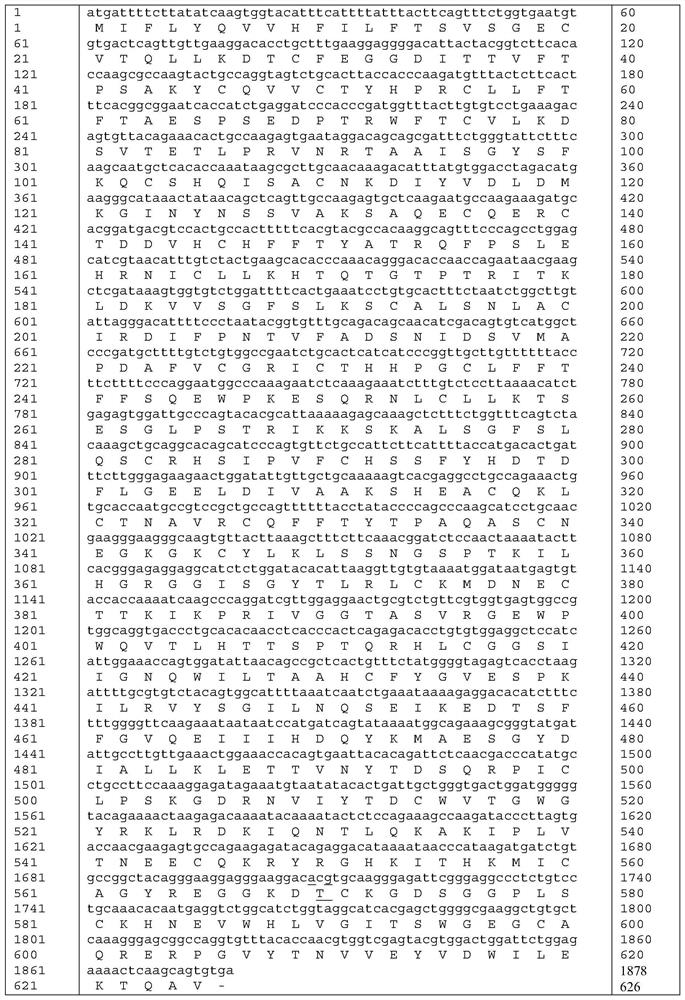
[0036] The amino acid sequence of the mutant protein of highly active blood coagulation factor XI mutant Ala570Thr is shown in SEQ ID NO:5.
[0037] The preparation method of the mutant protein of highly active coagulation factor XI mutant Ala570Thr comprises the following steps:
[0038](1) The human blood coagulation factor XI coding gene of human wild type or blood coagulation factor XI mutant Ala570Thr is connected in the vector to obtain the recombinant vector; (see Figure 6 )
[0039] (2) Transforming the above-mentioned recombinant vector into a host cell to obtain recombinant expression cell clones;
[0040] (3) Cultivating the above-mentioned cell clones in serum-free medium, expressing the expression of the mutant protein of highly active blood coagulation factor XI mutant Ala570Thr;
[0041] The serum-free medium is "SAFC Biosciences EX-CELL TM 302” (commercialized reagent). In order to ensure product safety and prevent blood-derived preparations from spreading...
Embodiment 2
[0054] Detection of thromboelastogram (see Figure 9)
[0055] Thromboelastogram (TEG): is a comprehensive test used to monitor the overall coagulation process in whole blood. It does not require blood sample processing, and uses a small amount of whole blood to monitor the interaction between coagulation factors, platelets, fibrinogen, fibrinolytic system and other cellular components, and accurately provide the patient's coagulation profile. When testing, first add anticoagulant blood to the activation monitoring reagent bottle, then suck out a certain volume and add it to a special cylindrical cup (add CaCl in advance 2 ). The cup rotates at an angle of 4°45' at a constant speed of 1 cycle / 9s. The coagulation state of the blood is monitored through a needle immersed in the blood and suspended by a helical wire, and the coagulation speed and intensity curve is drawn by the computer. The blood coagulation process is mainly evaluated through the following curve parameters: (1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com