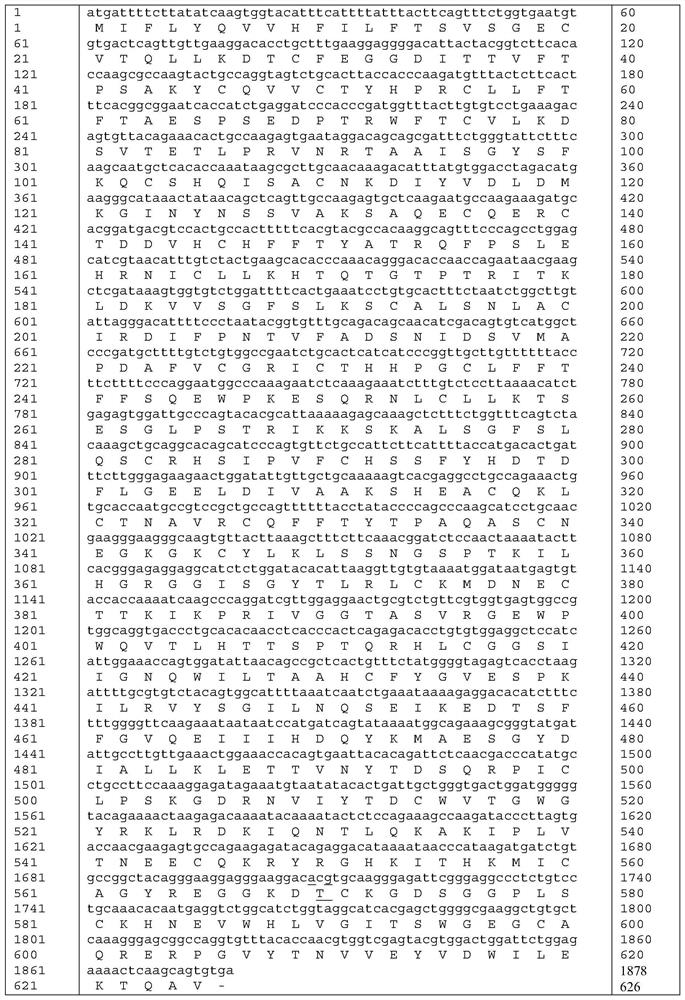
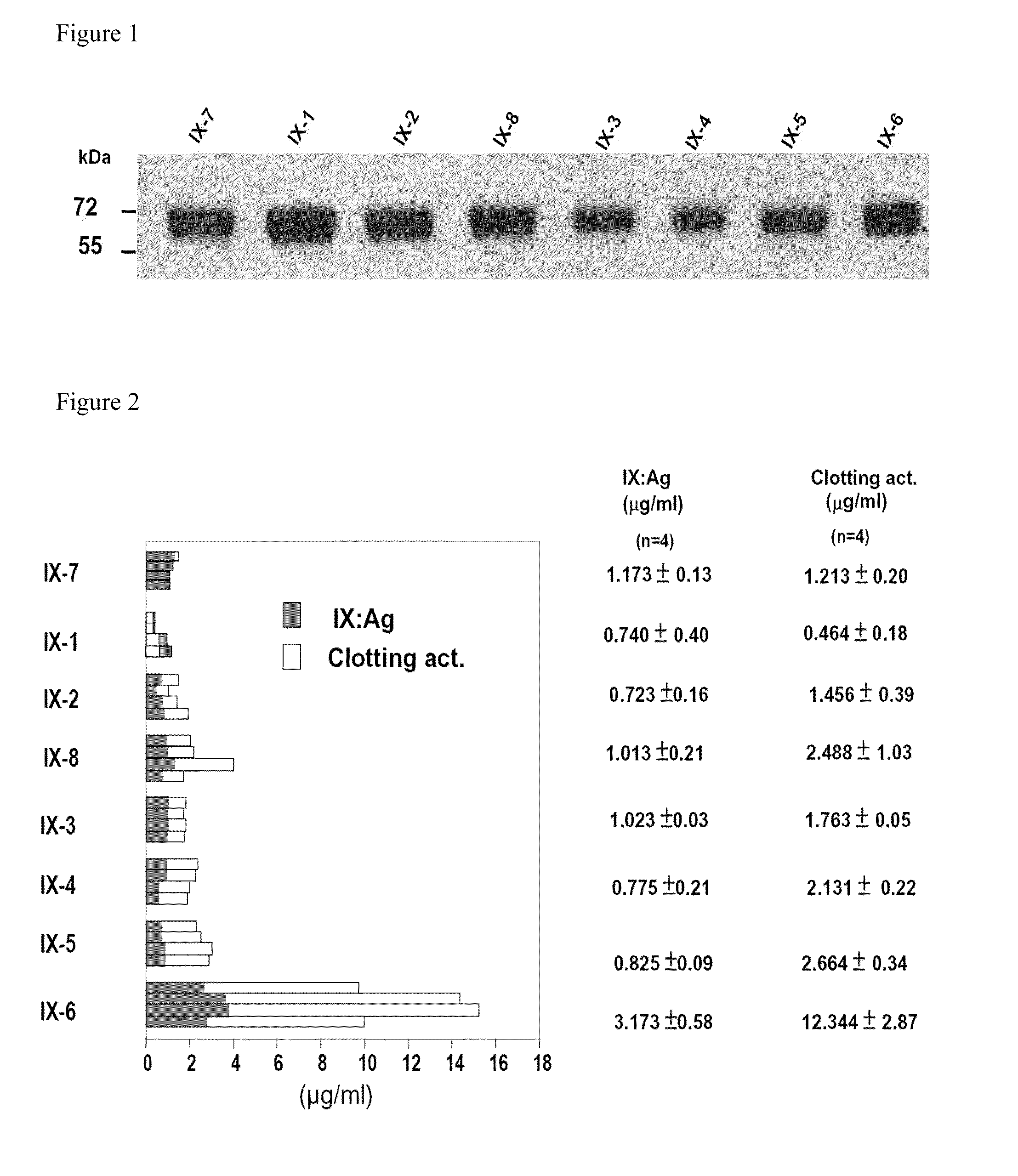
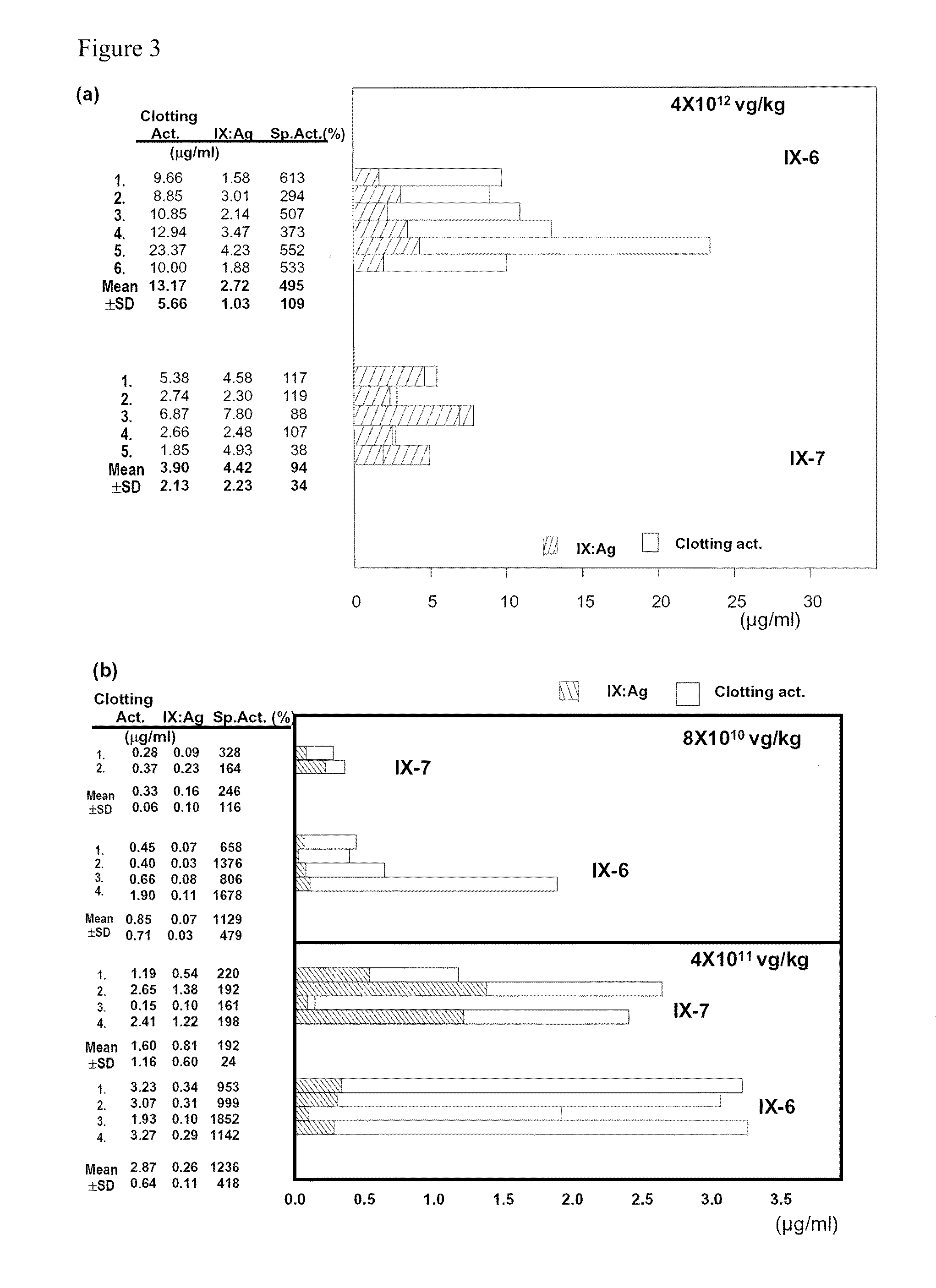
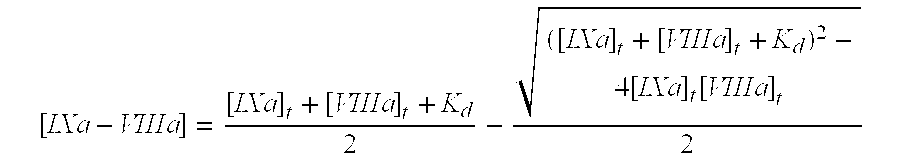Patents
Literature
32 results about "Increased Coagulation Activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
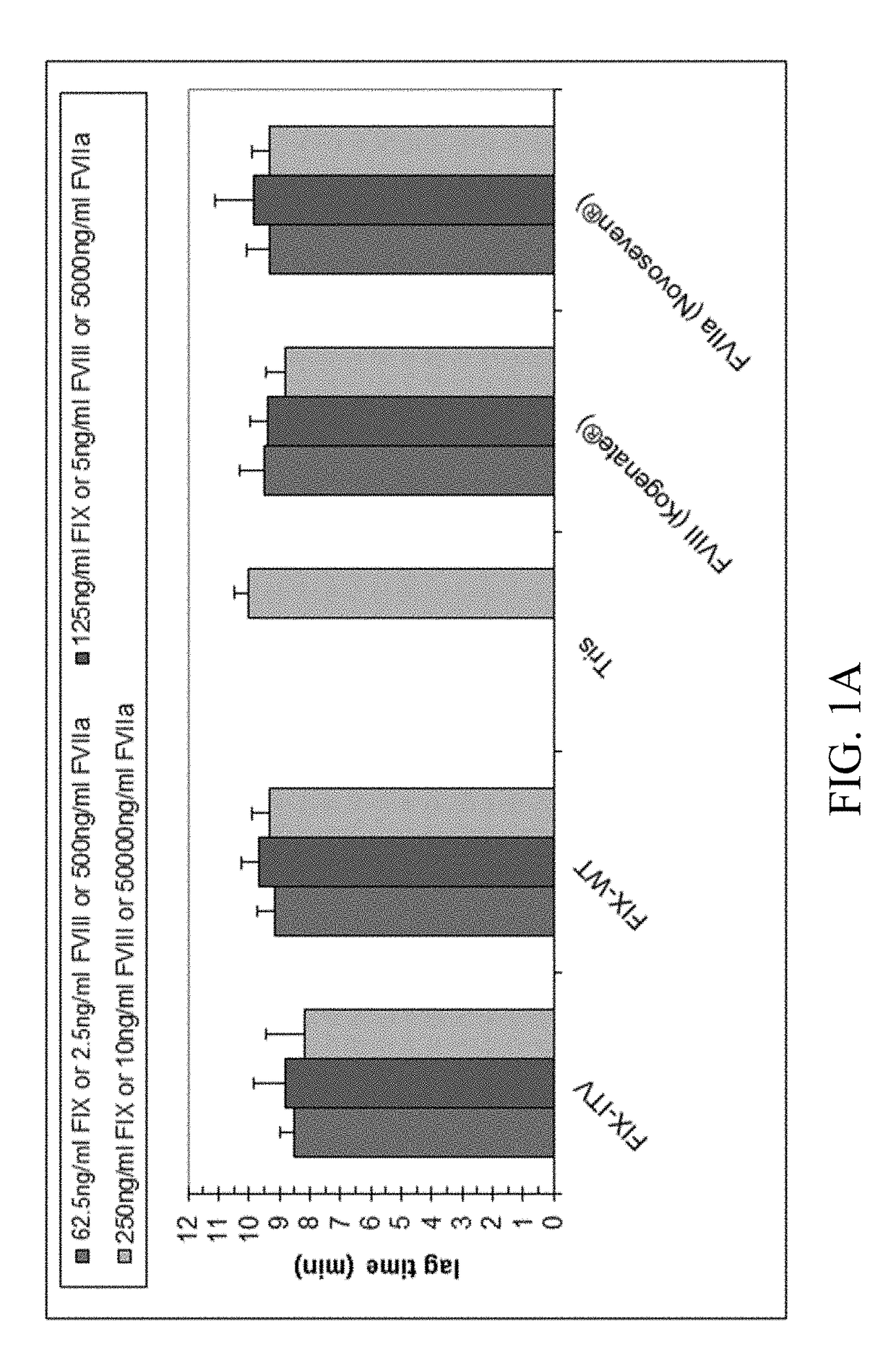
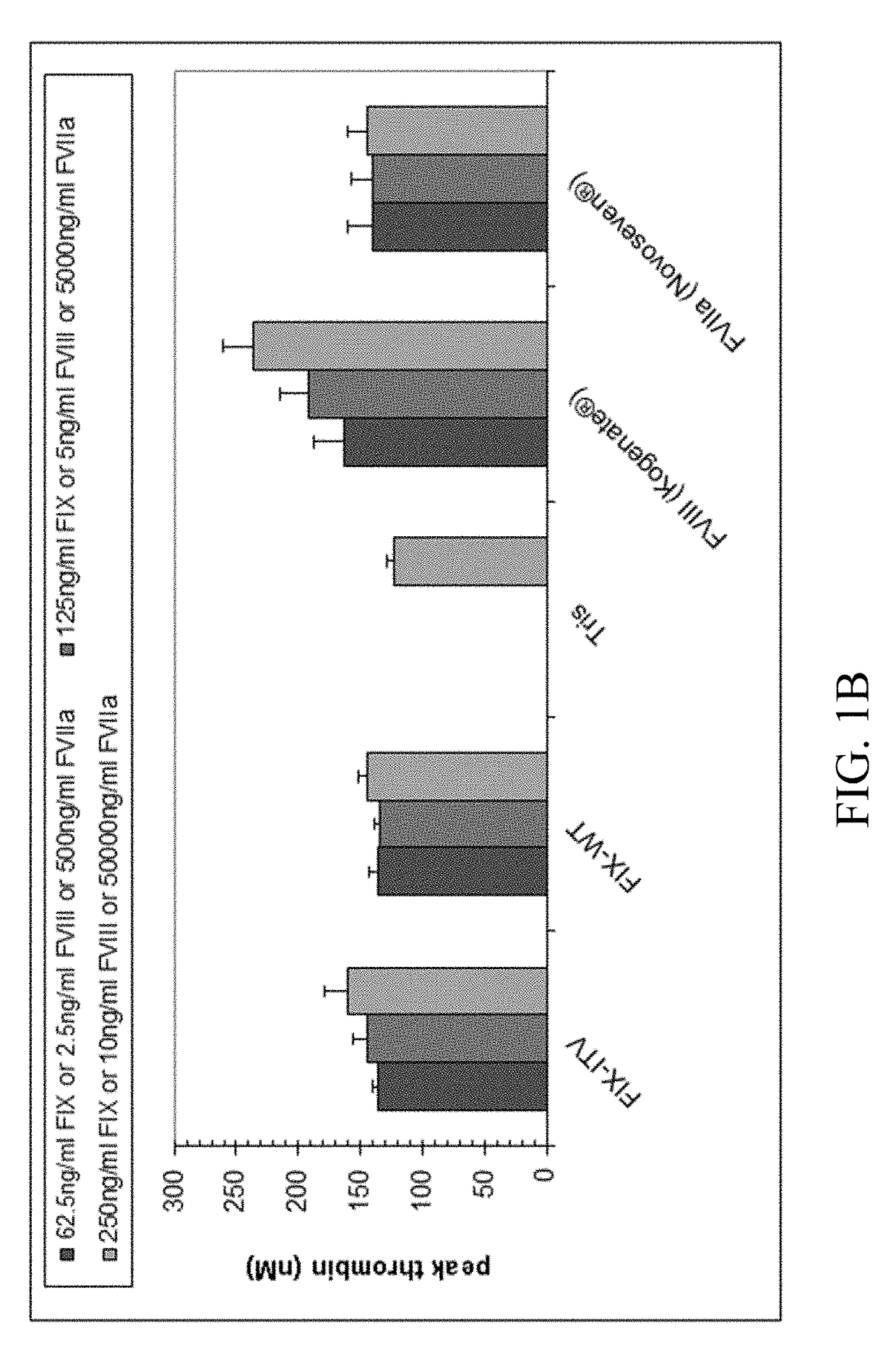
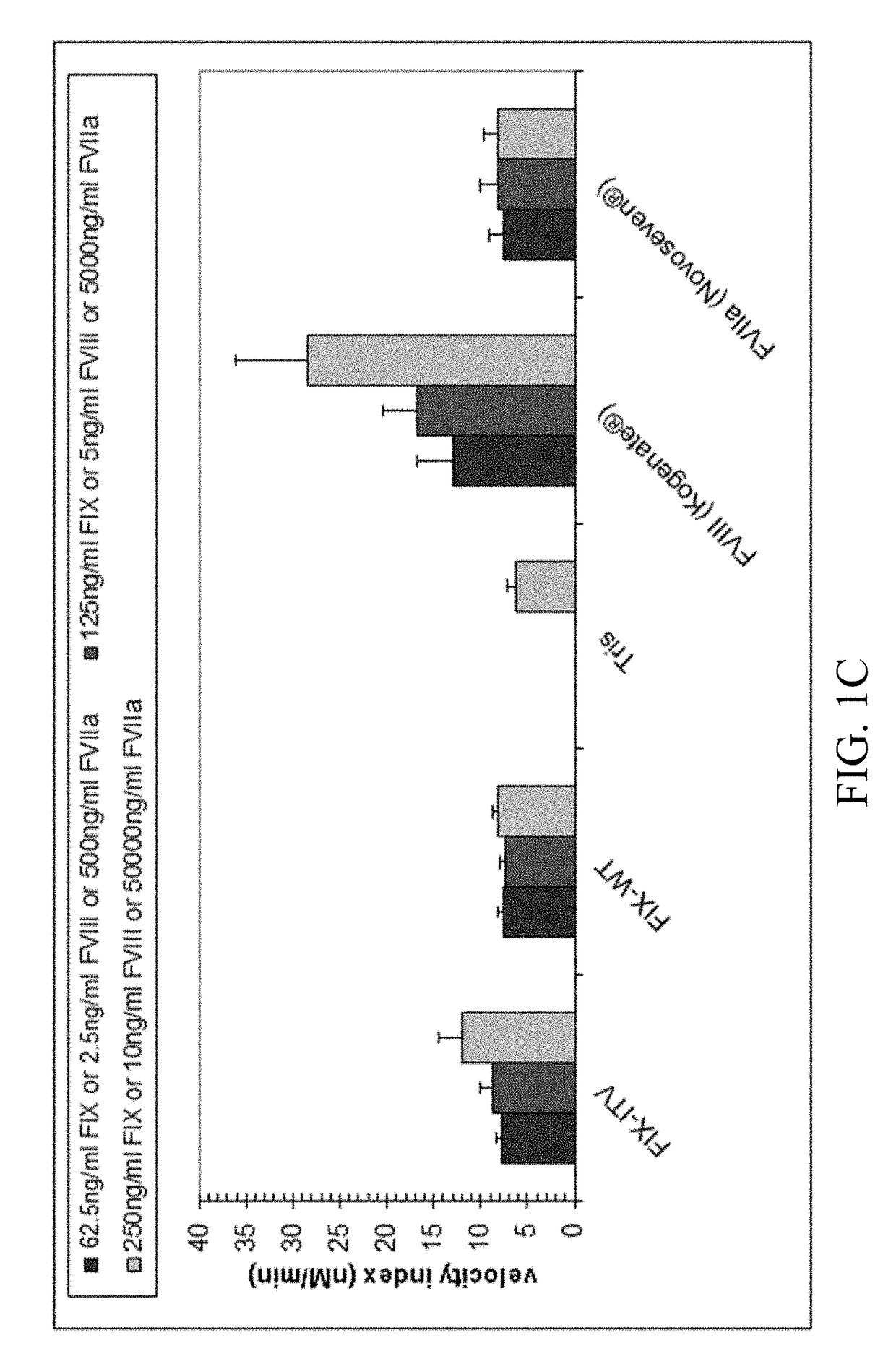
The association with F5 expression represents a possible link to the increased coagulation activity in breast cancer, and F10 rs3093261, located between the F10 and F7 gene, has been associated with increased FVII levels in stroke patients , which may link F10 rs3093261 to the increased coagulation activation observed in our study.
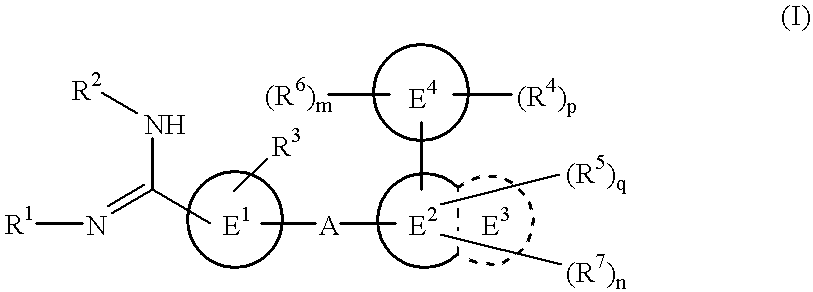
Amidino derivatives and drugs containing the same as the active ingredient
InactiveUS6358960B1BiocideGroup 5/15 element organic compoundsExtracorporeal circulationDisseminated coagulopathy
The novel amidino derivatives of the formula (I):wherein all the symbols are as in specification defined;have an inhibitory activity of a blood coagulation factor VIIa and are useful for treatment and / or prevention of several angiopathy caused by enhancing a coagulation activity, such as disseminated intravascular coagulation, coronary thrombosis, cerebral infarction, cerebral embolism, transient ischemic attack, cerebrovascular disorders, pulmonary vascular diseases, deep venous thrombosis, peripheral arterial obstruction, thrombosis after artificial vascular transplantation and artificial valve transplantation, post-operative thrombosis, reobstruction and restenosis after coronary artery bypass operation, reobstruction and restenosis after PTCA or PTCR, thrombosis by extracorporeal circulation and procoagulative diseases such as glomerlonephriitis.
Owner:ONO PHARMA CO LTD
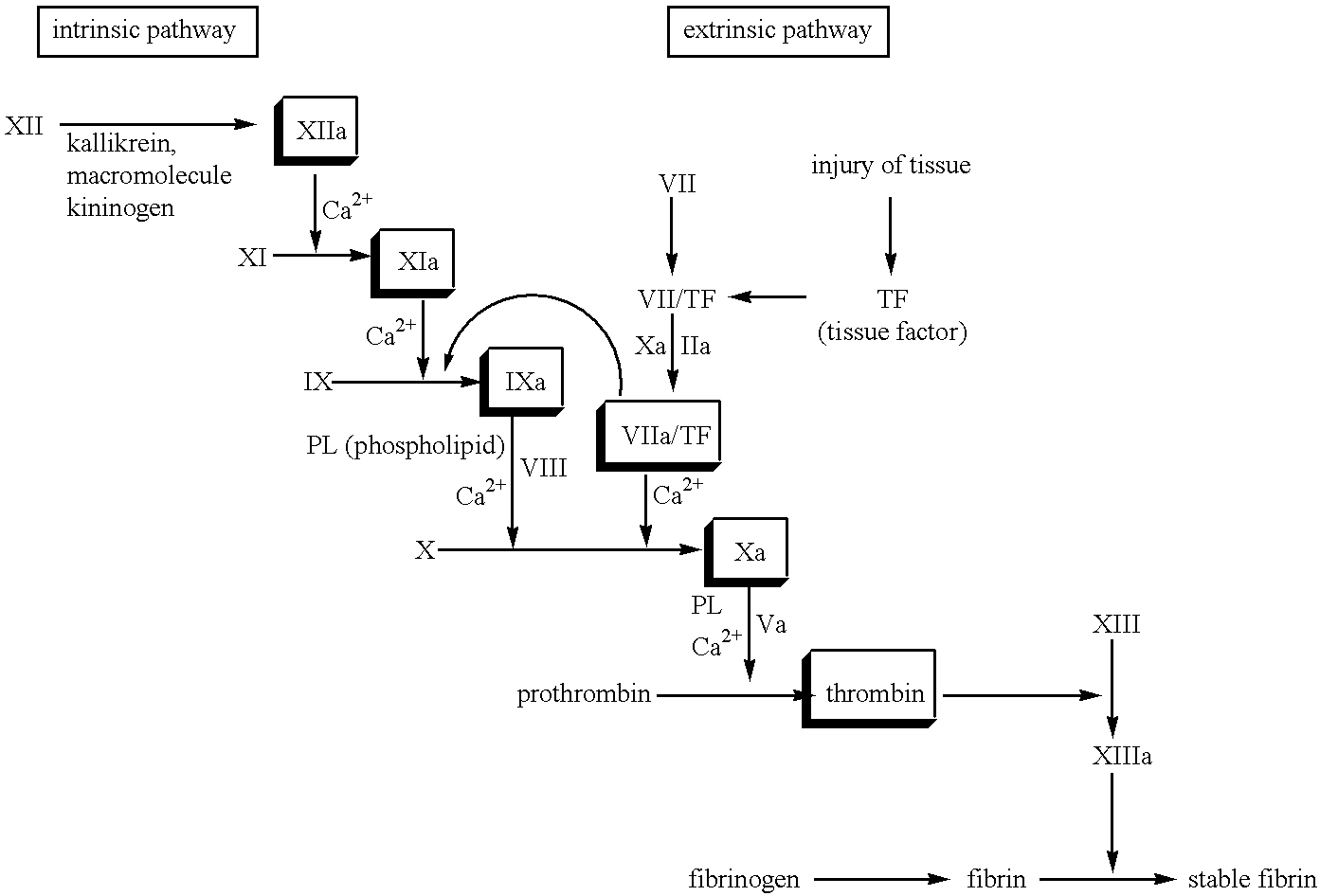
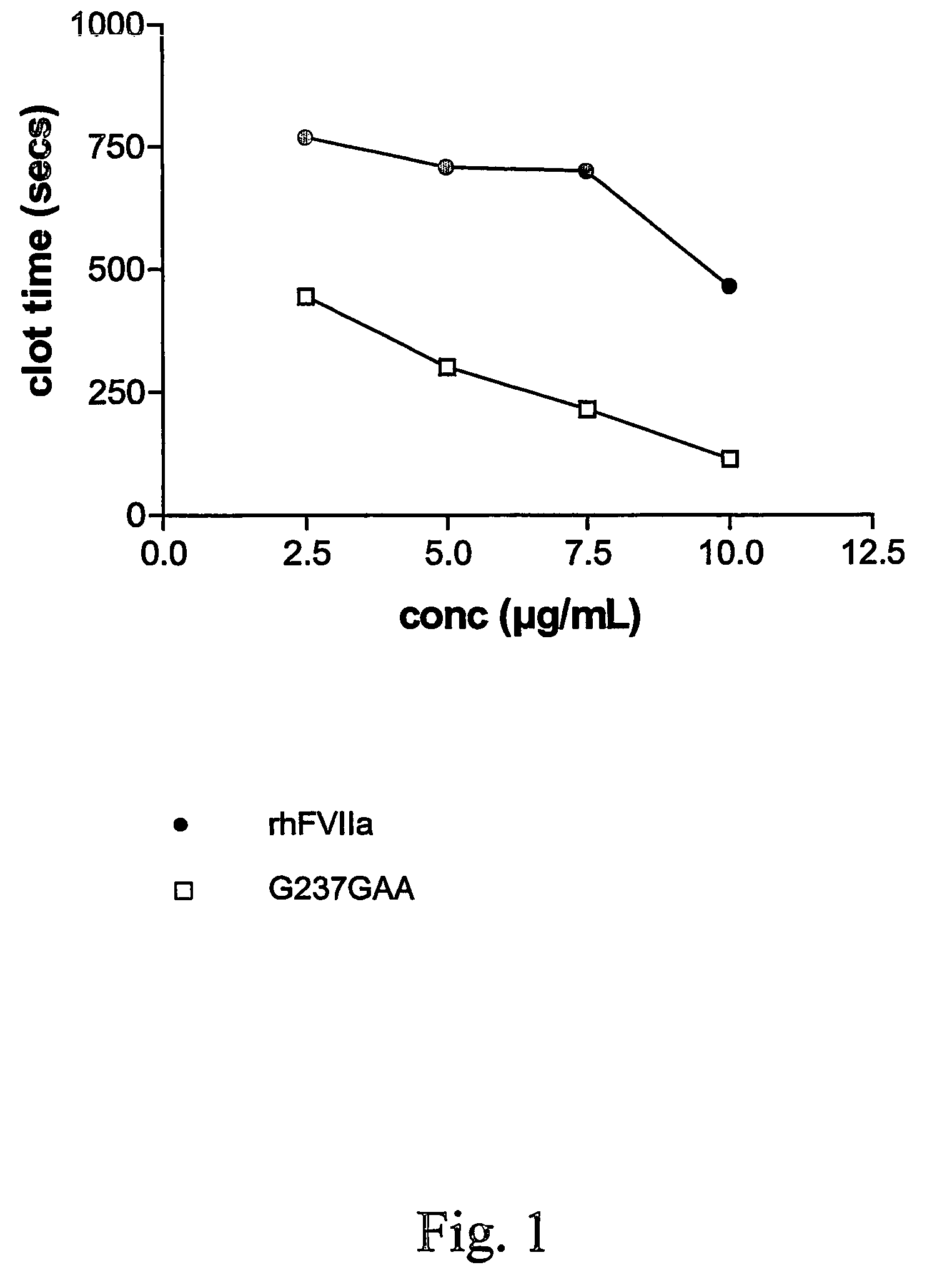
FVII or FVIIa variants
InactiveUS7771996B2Enhanced phospholipid membrane binding affinityProlong half-life in vivoPeptide/protein ingredientsImmunoglobulinsBiologyClotting time decreased
Variants of FVII or FVIIa comprising at least one amino acid modification in position 196, 237 or 341 relative to hFVII or hFVIIa. The variants exhibit an increased clotting activity, i.e. reduced clotting time, compared to rhFVIIa.
Owner:BAYER HEALTHCARE LLC
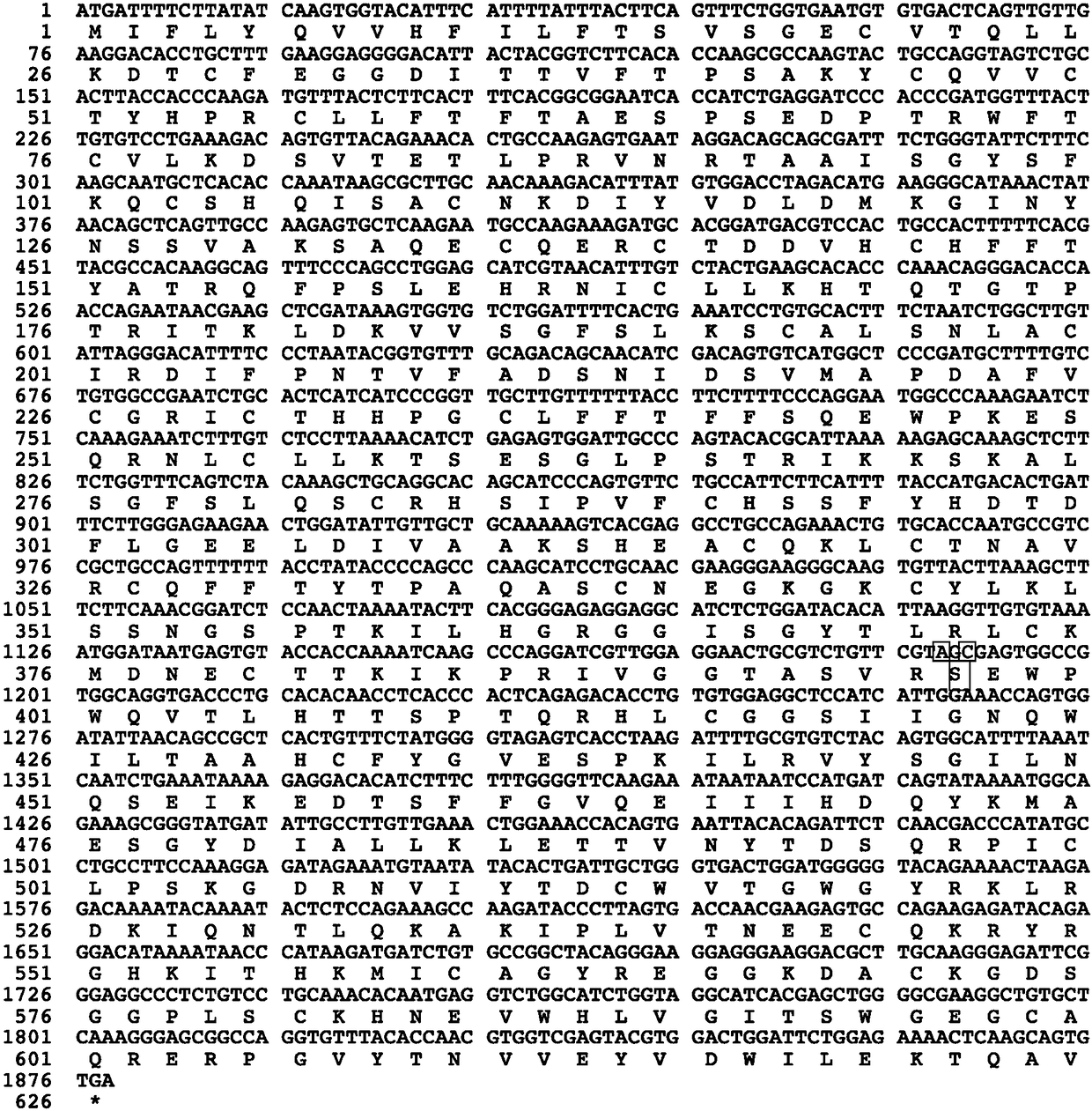
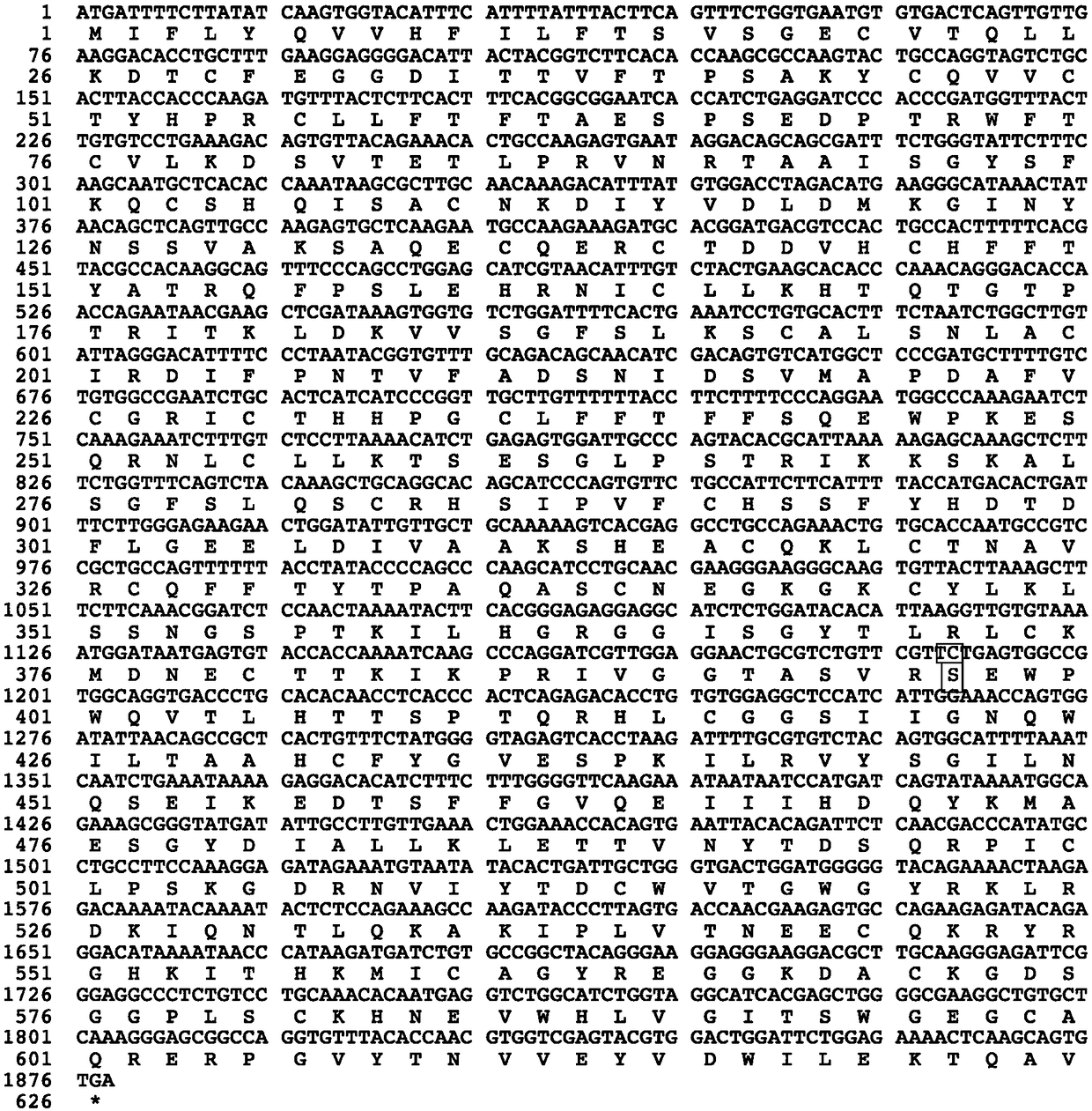
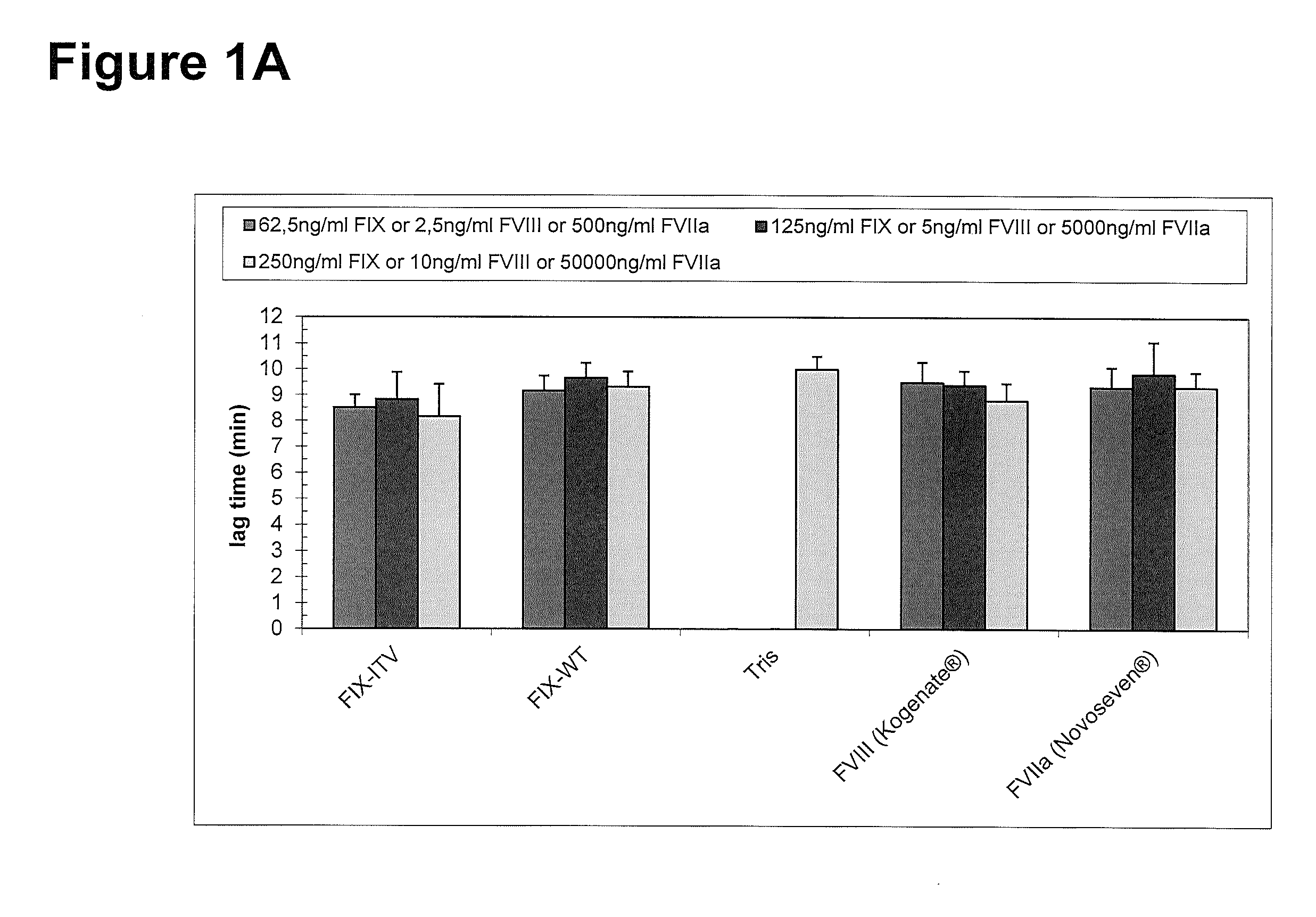
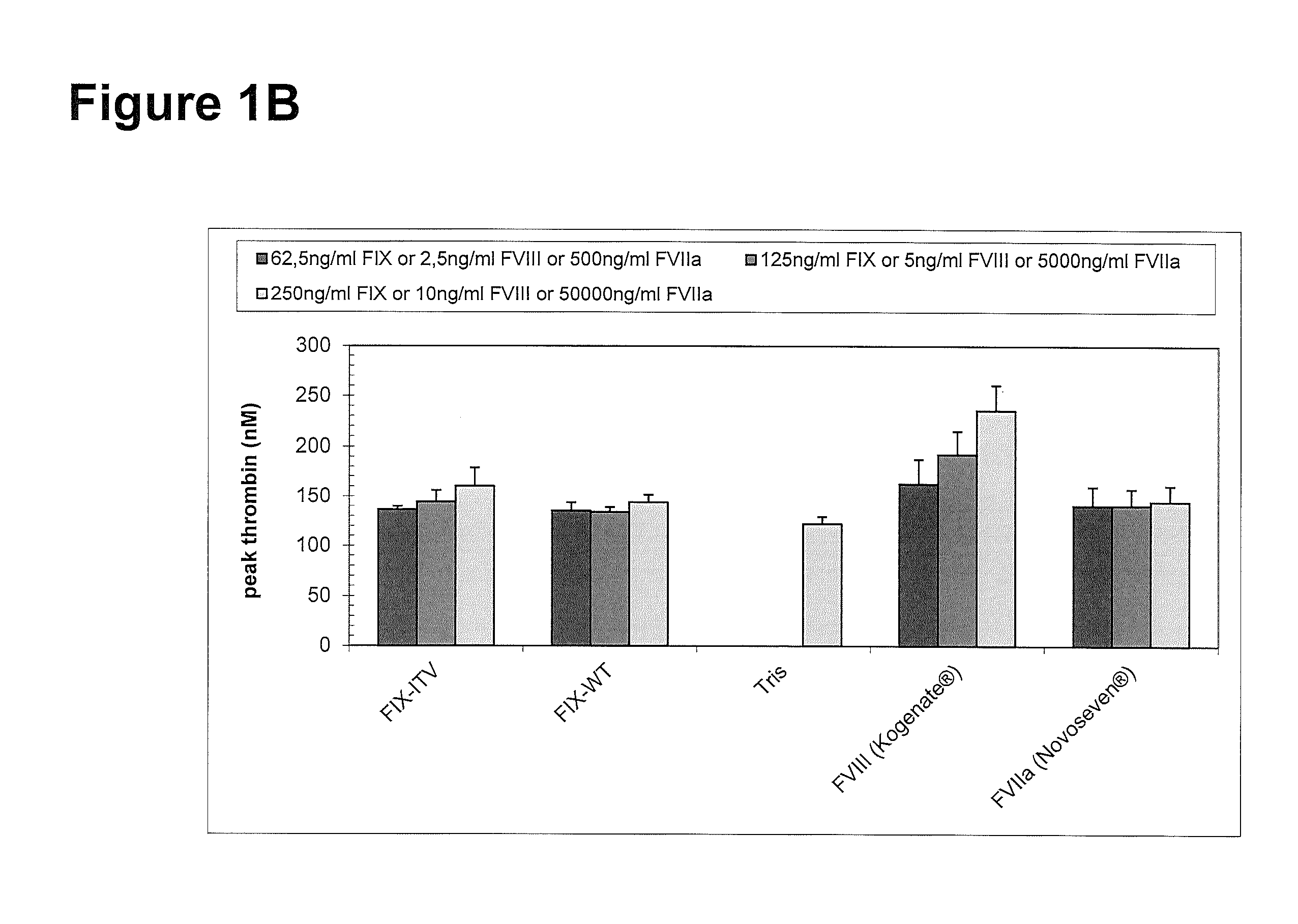
Factor IX variants with clotting activity in absence of their cofactor and/or with increased F.IX clotting activity and their use for treating bleeding disorders
The present invention relates to variants of factor IX (F.IX) or activated factor IX (F.IXa), wherein the variant is characterized in that it has clotting activity in absence of its cofactor. The present invention furthermore relates to variants of factor IX (F.IX) or activated factor IX (F.IXa), wherein the variant is characterized in that it has increased F.IX clotting activity compared to wildtype. The present invention furthermore relates to the use of these variants for the treatment and / or prophylaxis of bleeding disorders, in particular hemophilia A and / or hemophilia B or hemophilia caused or complicated by inhibitory antibodies to F.VIII. The present invention also relates to further variants of factor IX (F.IX) which have desired properties and can, thus be tailored for respective specific therapeutic applications.
Owner:DRK BLUTSPENDEDIENST BADEN WURTTEMBERG HESSEN GGMBH
High-activity blood coagulation factor XI mutant and preparation and application of gene therapy/editing vector and recombinant/fusion protein thereof
ActiveCN108220274AImprove coagulation functionGood prospects for alternative treatmentsPeptide/protein ingredientsNucleic acid vectorMutantOrganism
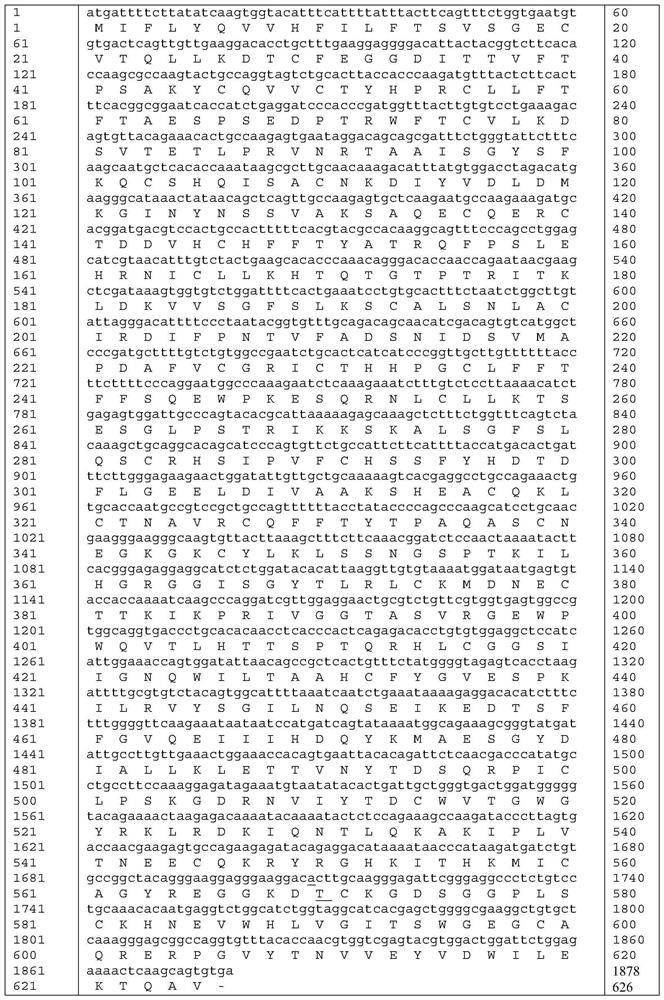
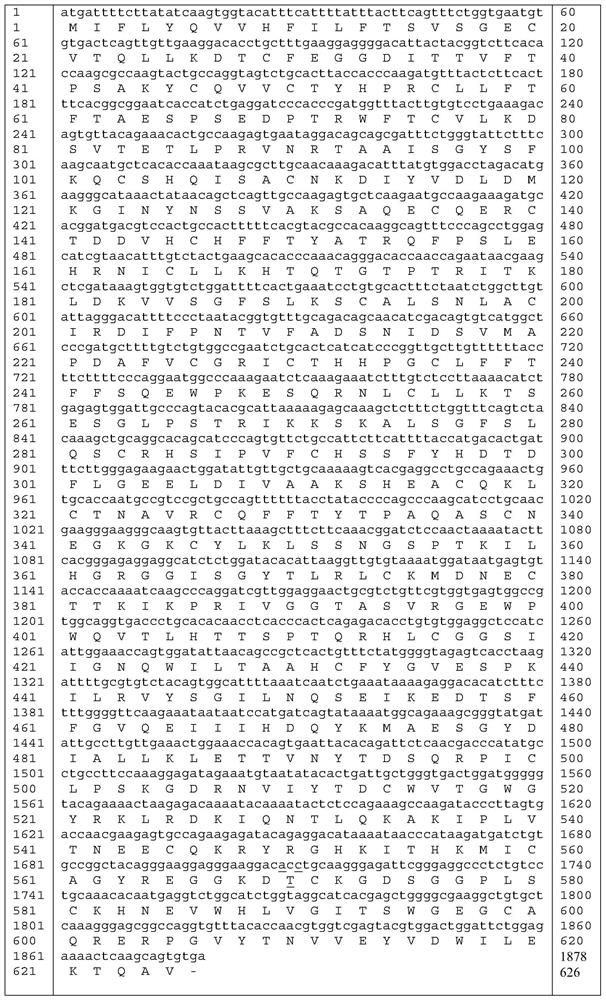
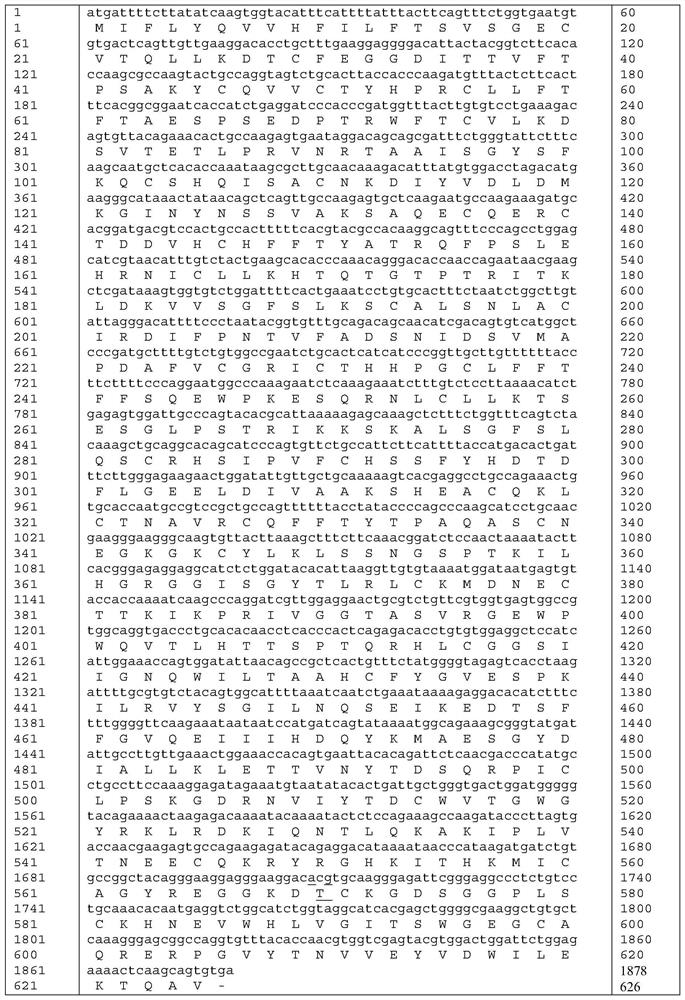
The invention relates to a high-activity blood coagulation factor XI mutant and preparation and application of a gene therapy / editing vector and recombinant / fusion protein thereof. A nucleotide sequence is as shown in SEQ ID NO:1-6, and an amino acid sequence is as shown in SEQ ID NO:7. The mutant provided by the invention has very high blood coagulation activity, can efficiently activate blood coagulation reaction and improve the whole blood coagulation function of the organism, is applied to therapy of hemorrhagic diseases, and has very good gene therapy, gene editing and recombinant proteinreplacement therapy prospects.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE +3
Factor IX Variants with Clotting Activity in Absence of Their Cofactor and/or With Increased F.IX Clotting Activity and Their Use for Treating Bleeding Disorders
The present invention relates to variants of factor IX (F.IX) or activated factor IX (F.IXa), wherein the variant is characterized in that it has clotting activity in absence of its cofactor. The present invention furthermore relates to variants of factor IX (F.IX) or activated factor IX (F.IXa), wherein the variant is characterized in that it has increased F.IX clotting activity compared to wildtype. The present invention furthermore relates to the use of these variants for the treatment and / or prophylaxis of bleeding disorders, in particular hemophilia A and / or hemophilia B or hemophilia caused or complicated by inhibitory antibodies to F.VIII. The present invention also relates to further variants of factor IX (F.IX) which have desired properties and can, thus be tailored for respective specific therapeutic applications.
Owner:DRK BLUTSPENDEDIENST BADEN WURTTEMBERG HESSEN GGMBH
Use of non-catalytic form of heparanase and peptides thereof for reversing the Anti-coagulant effects of heparinoids
InactiveUS20110104140A1Peptide/protein ingredientsMicrobiological testing/measurementMedicineHeparinoids
The present invention relates to inhibition of heparinoids anti-coagulation activity by a non-active form of a eukaryotic endoglycosidase or any fragment or peptide thereof comprising at least one heparin-binding domain. More particularly, the invention provides compositions and methods for the inhibition of heparinoids anti-coagulation activity and for the treatment of coagulation related pathologic clinical conditions, using a non-active form of mammalian heparanase or peptides thereof comprising at least one heparin-binding domain.
Owner:HADASIT MEDICAL RES SERVICES & DEVMENT +1
Factor IX variants with clotting activity in absence of their cofactor and their use for treating bleeding disorders
The present invention relates to variants of a vitamin K-dependent serine protease of the coagulation cascade, preferably variants of factor IX (F.IX), wherein the variant is characterized in that it has clotting activity in absence of its cofactor. The present invention furthermore relates to the use of these variants for the treatment and / or prophylaxis of bleeding disorders, in particular hemophilia A and / or hemophilia B or hemophilia caused or complicated by inhibitory antibodies to F.VIII. The present invention also relates to further variants of factor IX (F.IX) which have desired properties and can, thus be tailored for respective specific therapeutic applications.
Owner:DRK BLUTSPENDEDIENST BADEN WURTTEMBERG HESSEN GGMBH
High-activity blood coagulation factor XI mutant Ala570Thr
ActiveCN112126636AImprove catalytic abilityHigh catalytic activityPeptide/protein ingredientsFermentationZymogenDisease
The present invention relates to a high-activity blood coagulation factor XI mutant Ala570Thr (A570T), nucleotide sequences are shown as SEQ ID NO: 1-4 and an amino acid sequence is shown as SEQ ID NO: 5. A zymogen state is activated into an enzyme with activity to produce resistance of a physiological inhibitor, thus the high-activity blood coagulation factor XI mutant Ala570Thr has very high coagulation activity and stronger catalytic capability on a non-physiological substrate, is applied to treatments of hemorrhagic diseases, and has very good prospects for gene treatment, gene editing andrecombinant protein replacement.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE +3
Recombinant human factor ix and use thereof
ActiveUS20100081712A1Easy to adaptOrganic active ingredientsPeptide/protein ingredientsFactor iiWild type
The present invention aims at converting factor IX into a molecule with enhanced activity which provides an alternative for replacement therapy and gene therapy for hemophilia B. Using recombinant techniques, factor IX with replacement at positions 86, 277, and 338 exhibits better clotting activity than recombinant wild type factor IX.
Owner:LIN SHU WHA
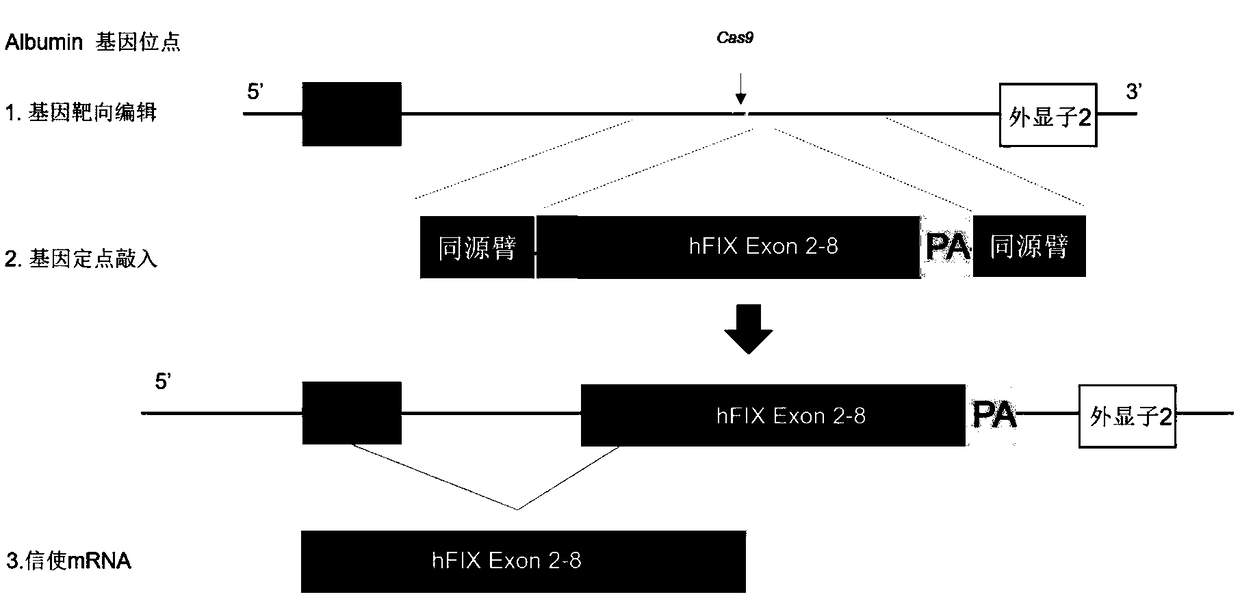
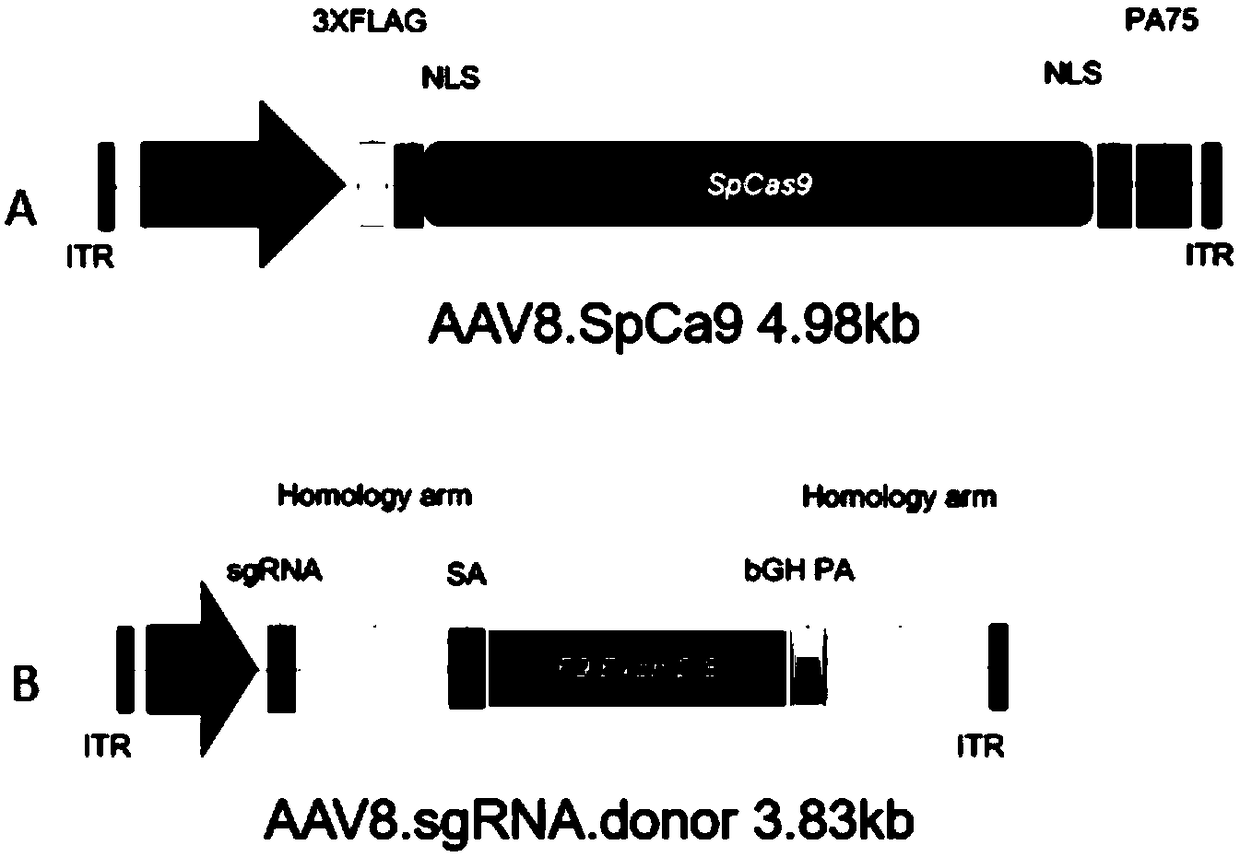
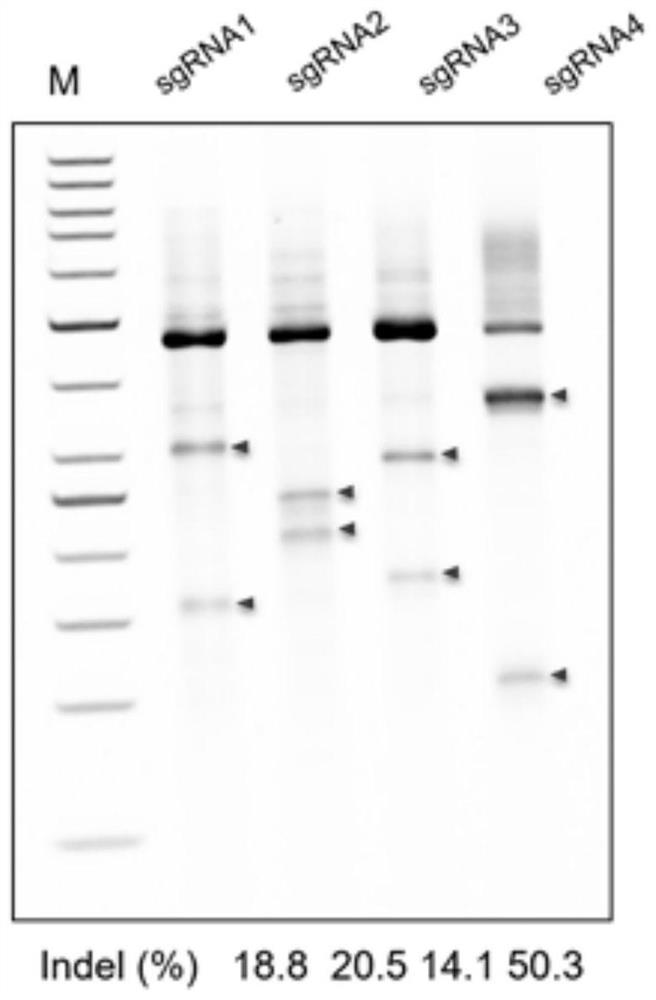
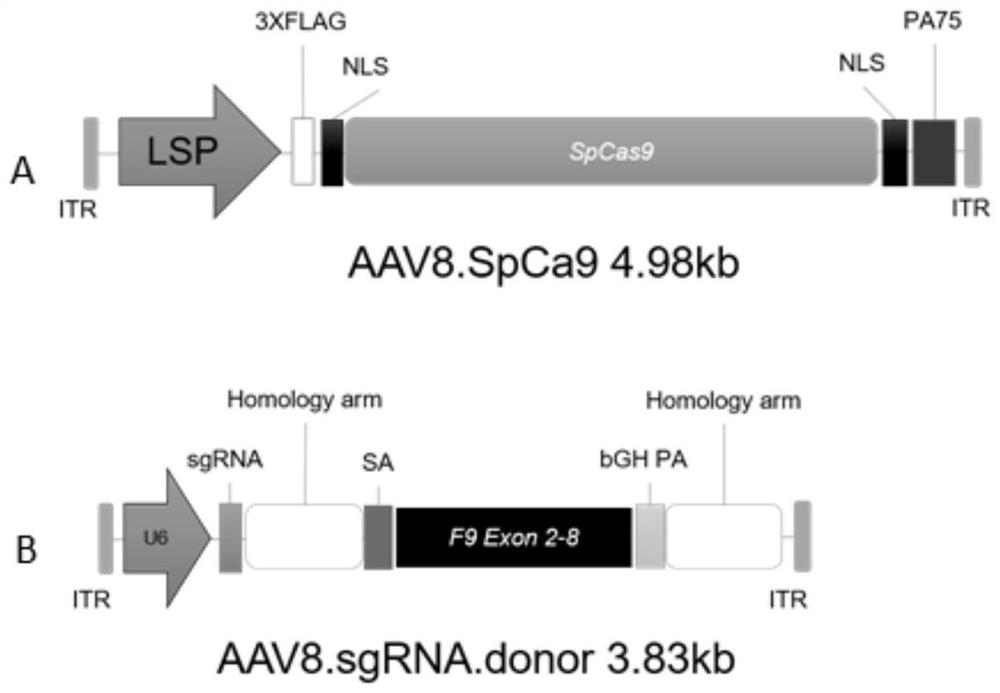
Composition for improving coagulation activity of patient with type B hemophilia, medicine and sgRNA (small guide Ribonucleic Acid)
ActiveCN108977464APeptide/protein ingredientsStable introduction of DNAB hemophiliaHemophilia patient
The invention discloses a composition for improving the coagulation activity of a patient with type B hemophilia, a medicine and sgRNA (small guide Ribonucleic Acid), and relates to the field of genetreatment of hemophilia. The composition comprises a first AAV (Adeno-associated Virus) vector and a second AAV vector for expressing the sgRNA; a core sequence of the sgRNA is selected from SEQ ID NO. 6 to SEQ ID NO. 9. The composition can be used for treating the type B hemophilia and recovering the coagulation activity.
Owner:成都金唯科生物科技有限公司
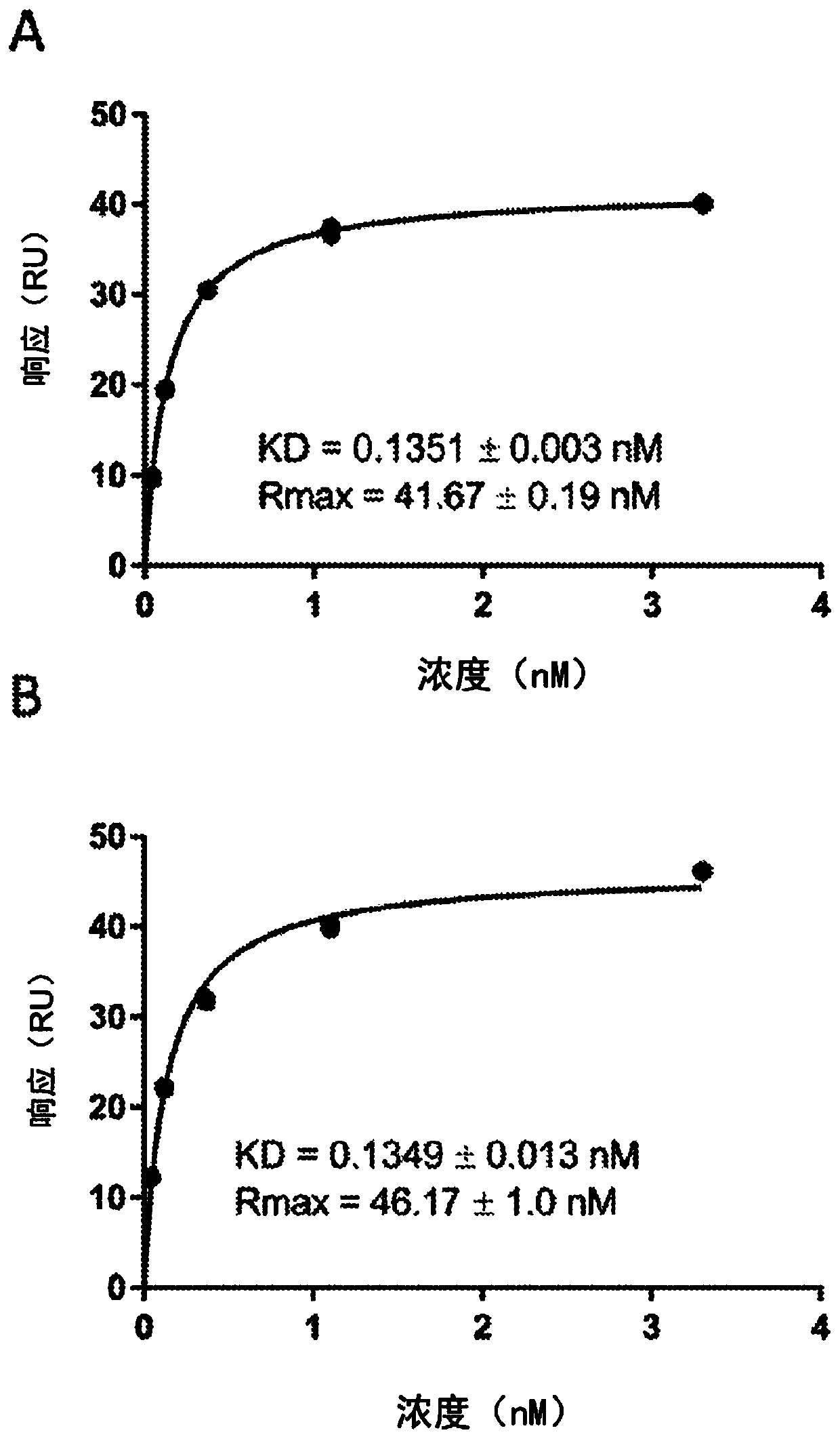
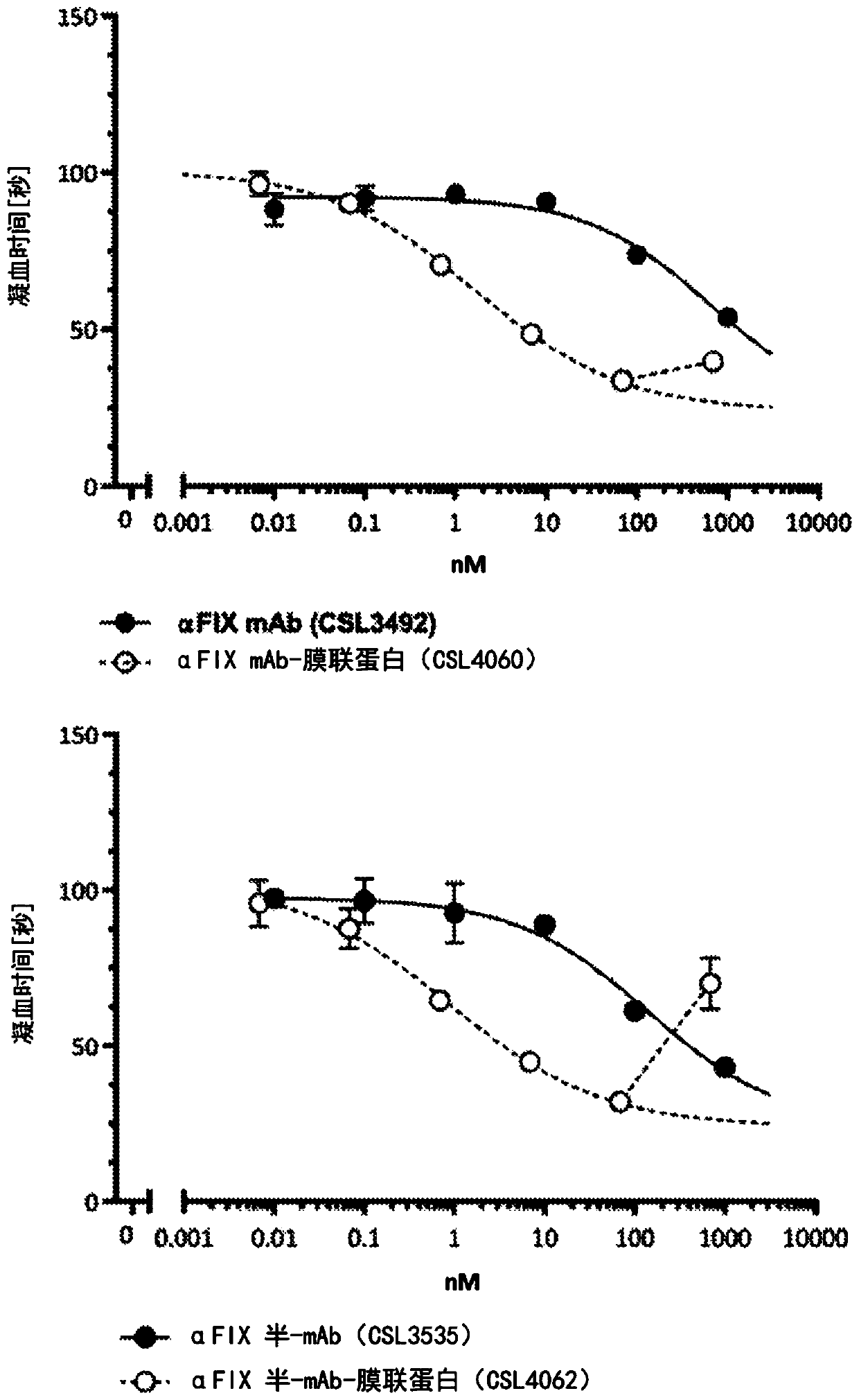
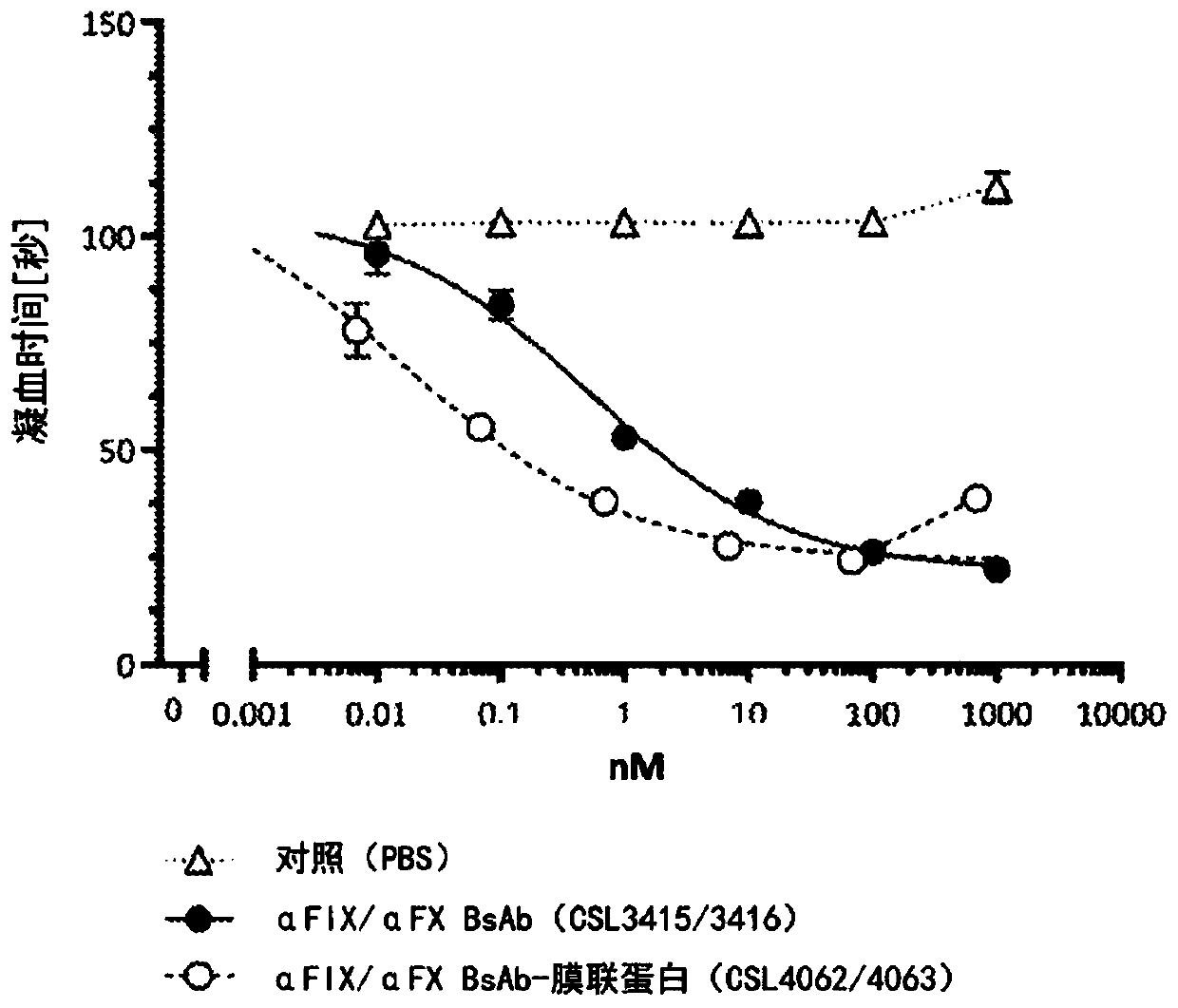
Coagulation factor binding proteins and uses thereof
InactiveCN110023339ASafe treatment optionsSmall doseImmunoglobulins against blood coagulation factorsFactor VIIClotting factorTarget binding
A membrane targeted binding protein that binds to at least one blood coagulation factor, wherein the binding protein has pro-coagulant activity.
Owner:CSL LTD
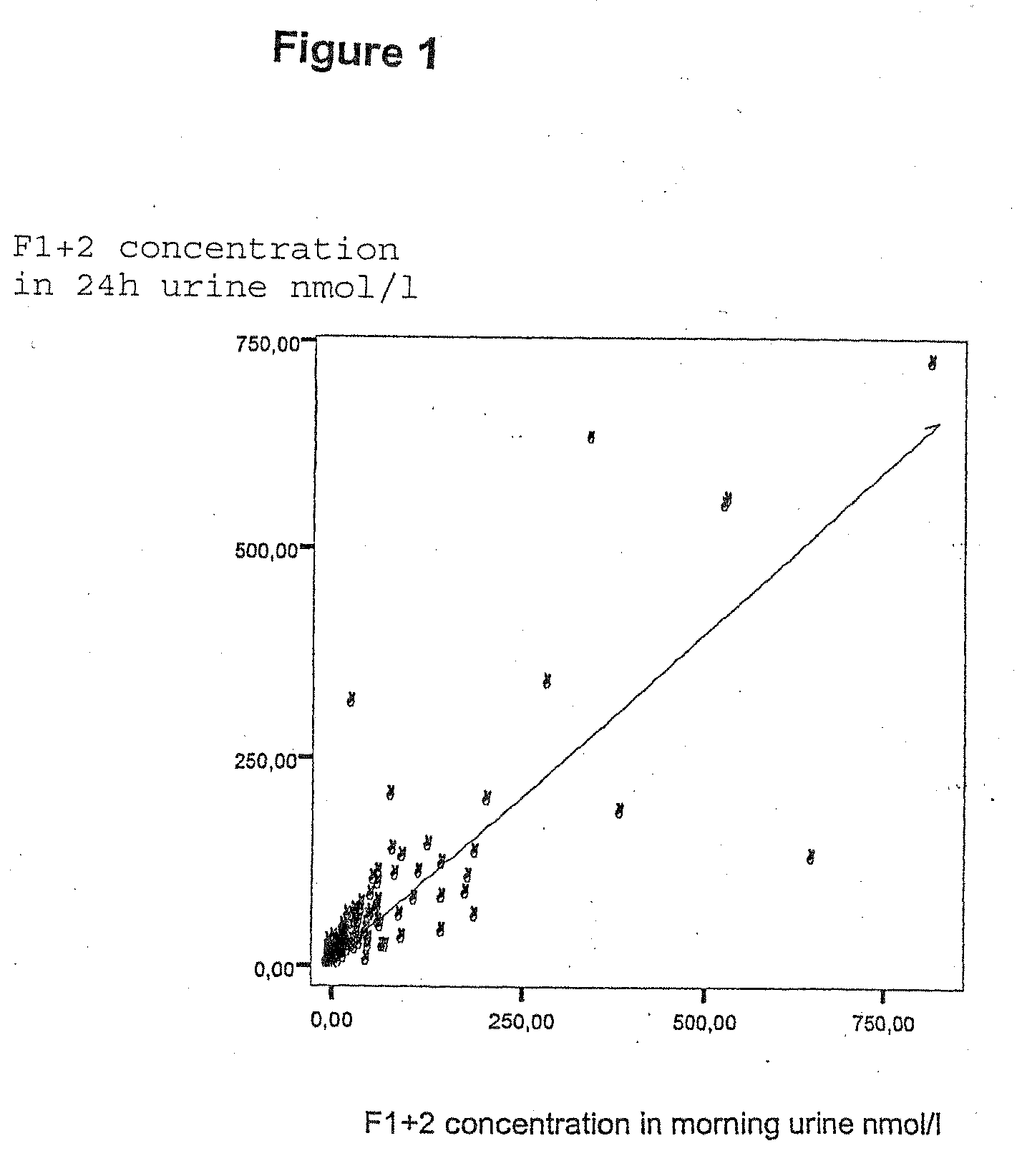
Detection of a blood coagulation activity marker in a body fluid sample
InactiveUS20100129841A1Shorten the time periodEasy to useImmunoglobulins against blood coagulation factorsMaterial analysis by observing effect on chemical indicatorBlood specimenHematological test
The invention relates to a method for detecting in a body fluid sample at least one blood coagulation activity marker that reflects the blood coagulation activity of an individual. By correlating the amount or concentration of the blood coagulation activity marker present e.g. in a urine sample, it is possible to monitor the blood coagulation activity of a patient following surgery without having to obtain a blood sample from said patient.
Owner:LASSEN MICHAEL RUD +1
Amino acid-substituted coagulation factor V
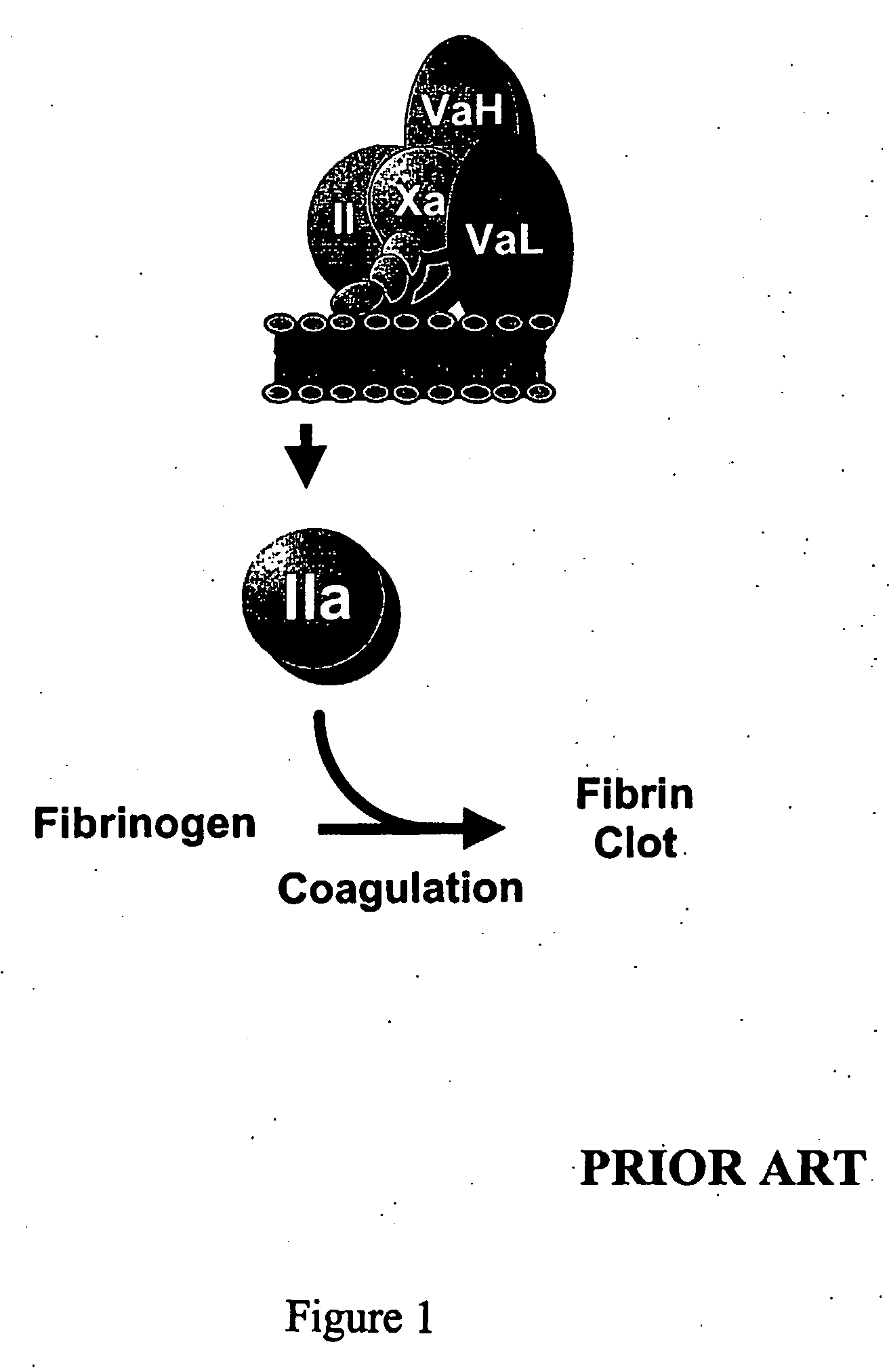
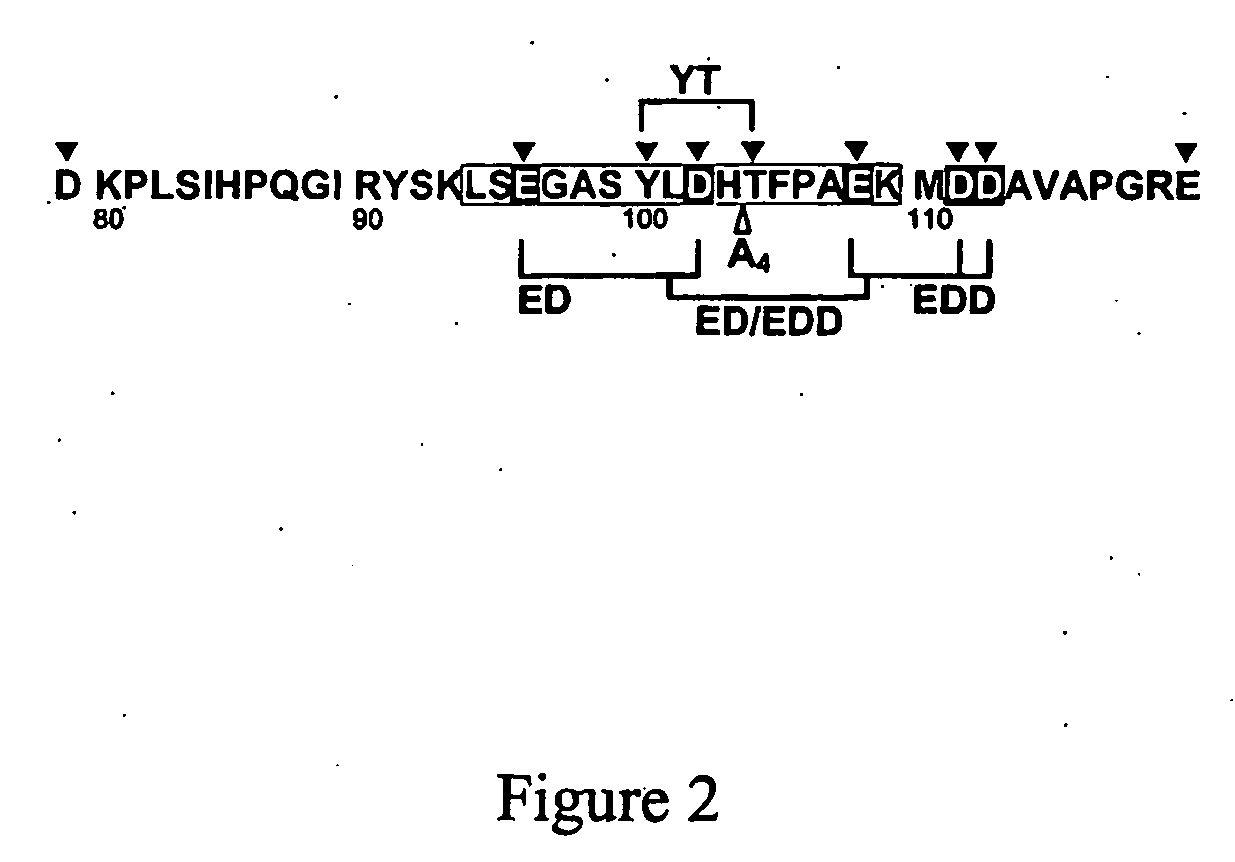
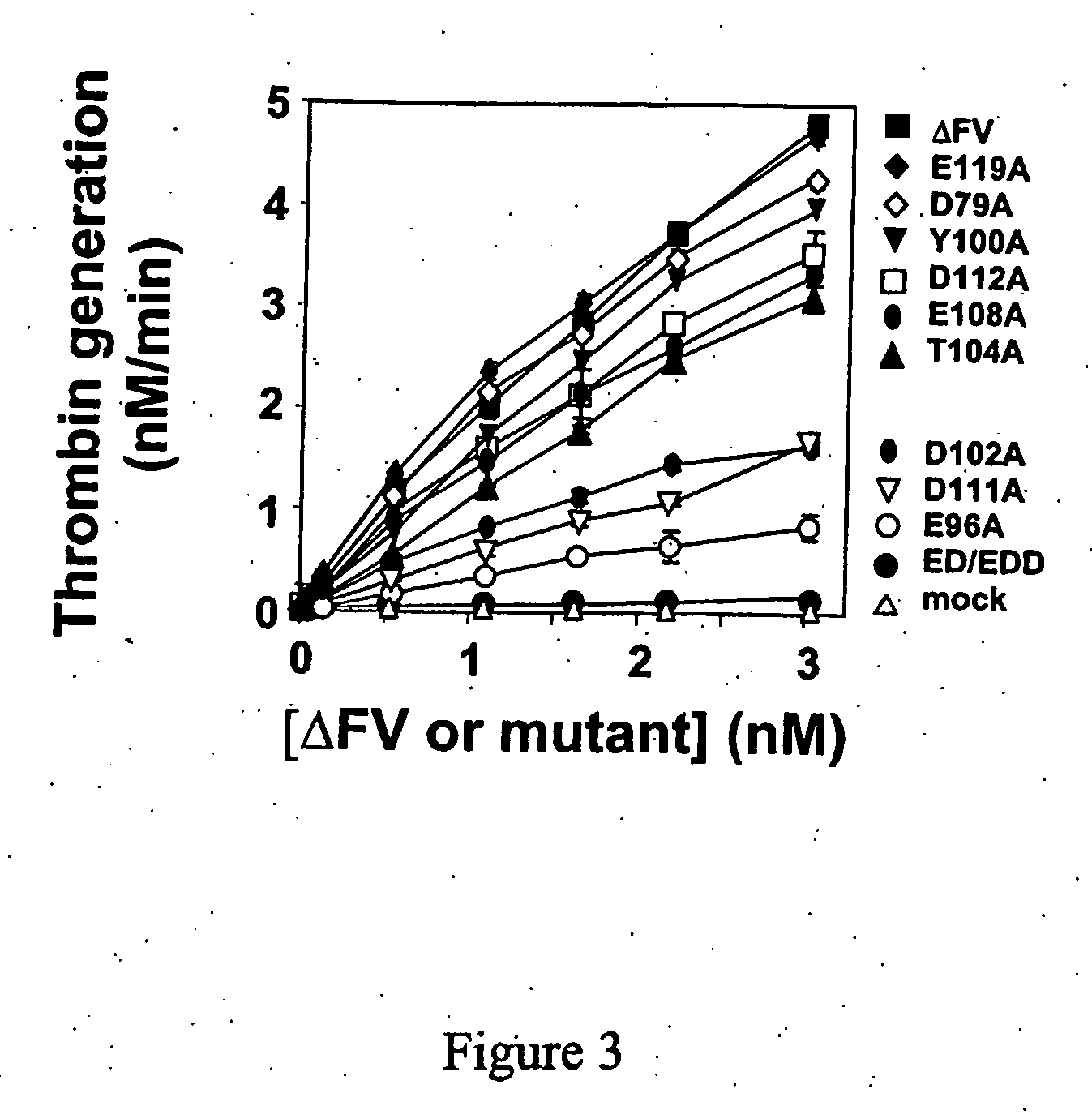
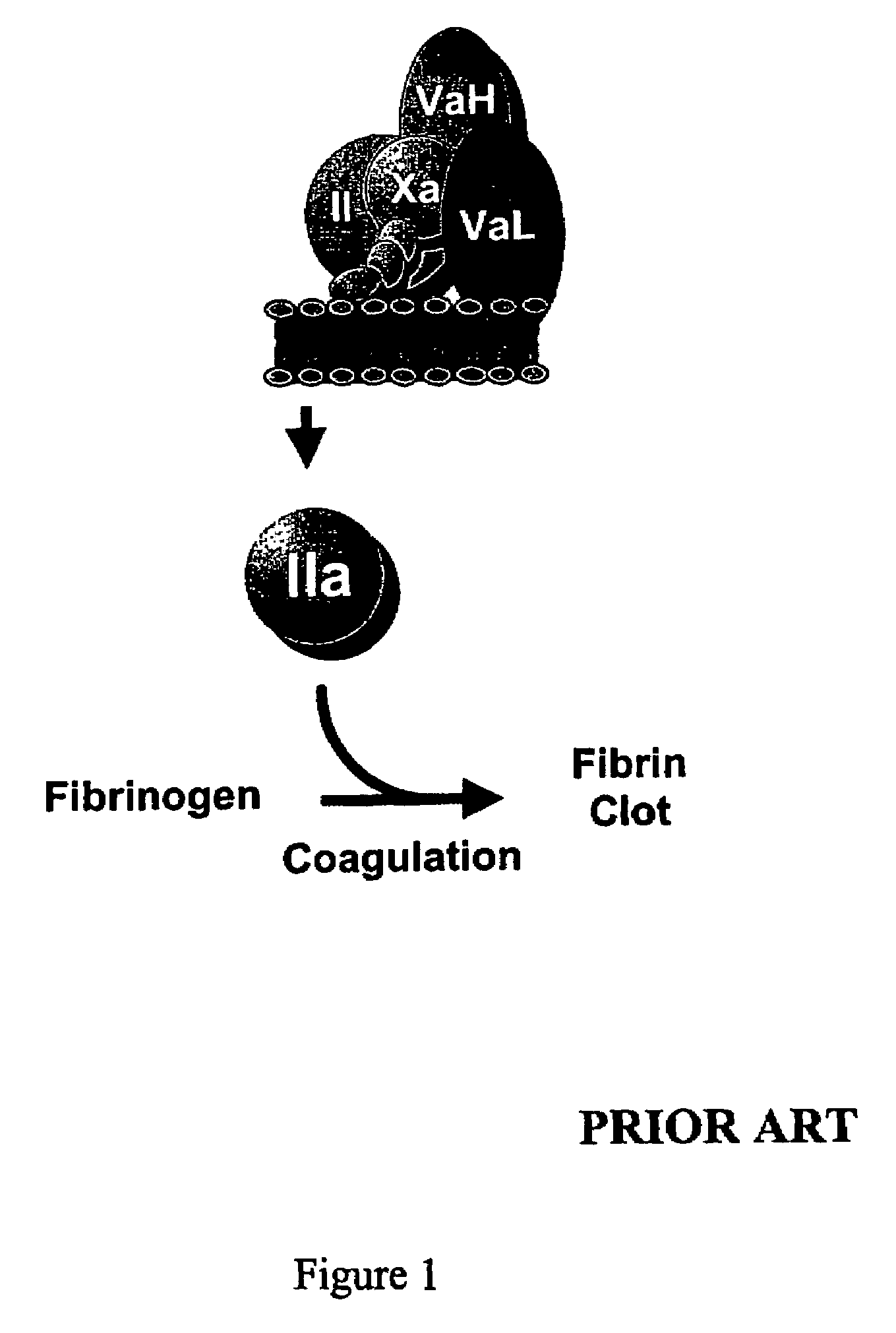
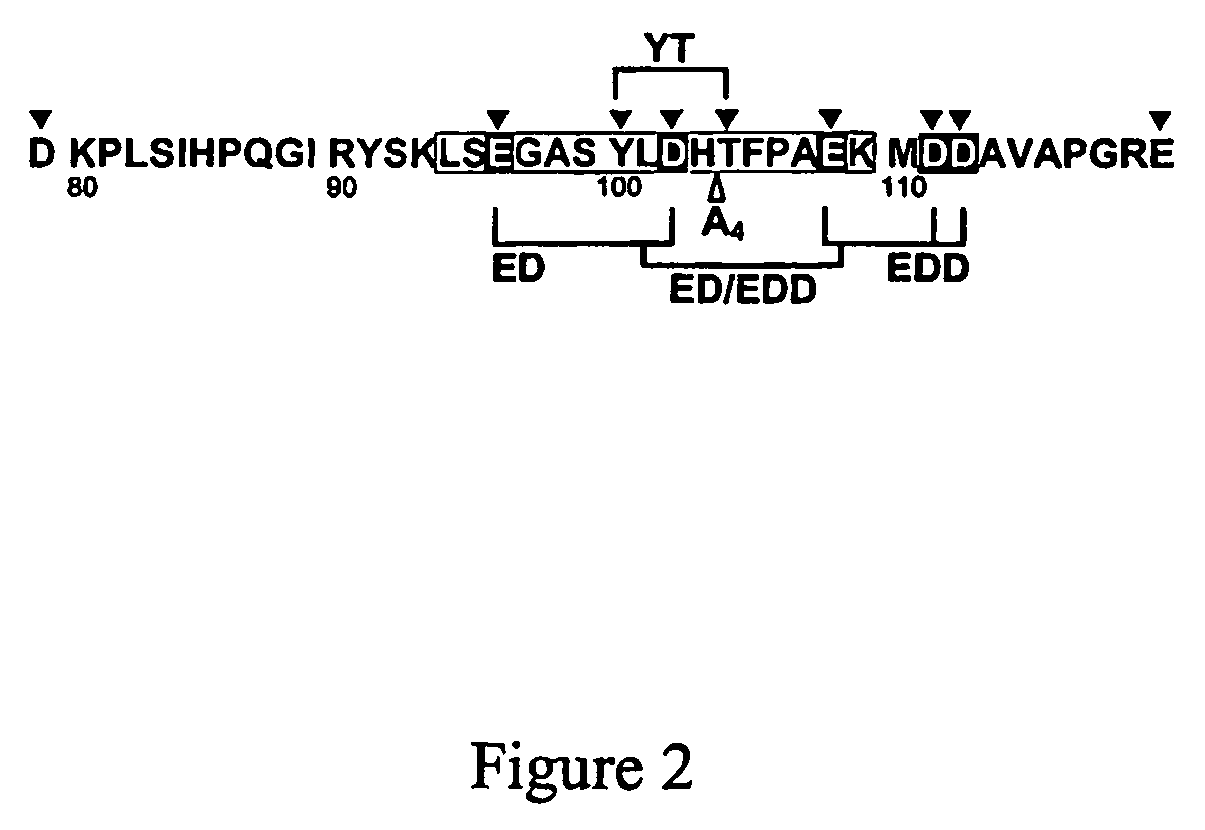
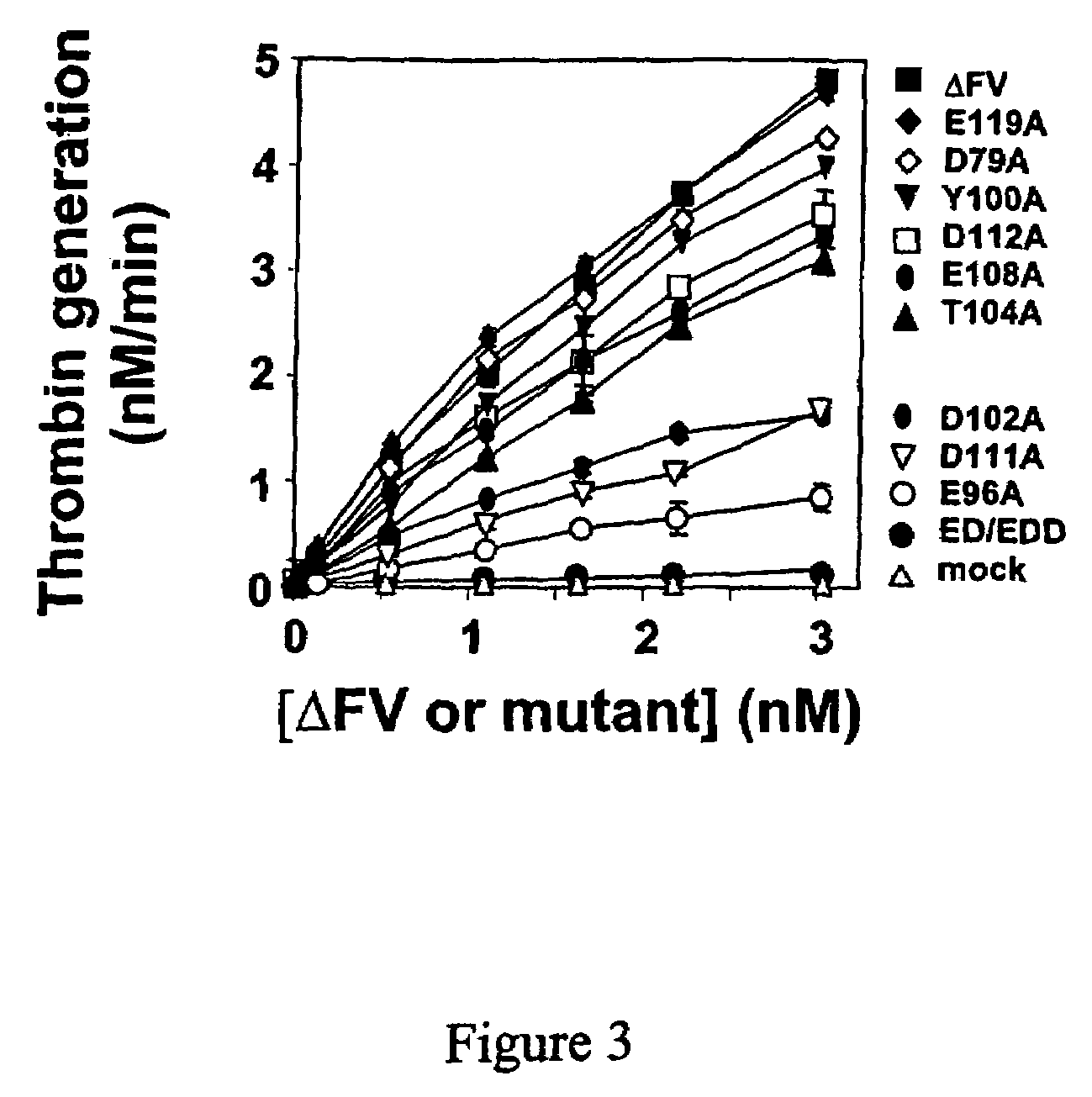
InactiveUS20060241039A1Reduces blood clotting activityReducing thrombin generationFibrinogenHydrolasesMedicineAmino acid substitution
There is provided FV derivatives that reduce blood clotting activity, by reducing thrombin generation, when compared to wild-type FV. In particular, the FV of the present invention comprises single-point and multi-point mutations, encompassed by aspartic acid 79 to glutamic acid 119. The derivatives can be used to treat patient with conditions necessitating reduced clotting activity.
Owner:CANADIAN BLOOD SERVICES
Blood compatible surfaces
InactiveUS20150093543A1Limit intrinsic coagulation activityIncrease the curvatureDiagnosticsSurgeryRough surfaceBlood compatible
The disclosure features blood compatible articles and methods of making the articles. The methods include providing a substrate and forming a rough surface on the substrate. The rough surface includes a plurality of three-dimensionally curved features each having a radius of curvature of less than about 50 nm. The surface includes a sufficient concentration of features per unit area to limit blood coagulation activity on the substrate and to limit the number of platelets that adhere to the surface when the substrate is exposed to blood.
Owner:TEIJIN LTD +1
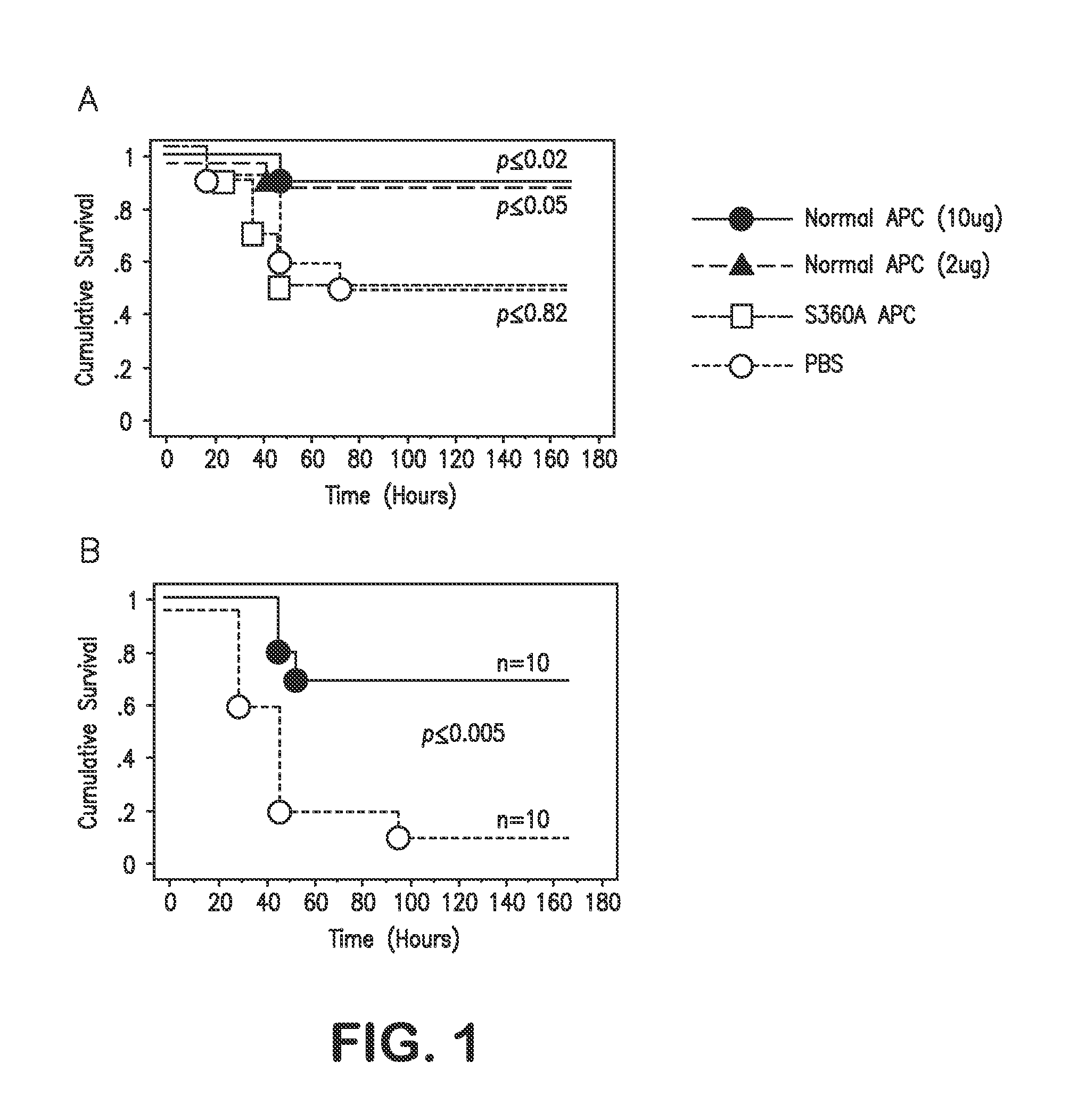
Dosing regimen of activated protein c and variants having reduced anticoagulant activity
InactiveUS20100284997A1Reduced anticoagulantReduce mortalityAntibacterial agentsHydrolasesDosing regimenLethal dose
Recombinant activated protein C (APC) and APC variants with reduced anticoagulant activity were used to reduce mortality in murine models of sepsis. These models included endotoxemia and bacteremia models. We discovered that single or multiple bolus doses of APC, especially of APC variants such as RR230 / 231AA-APC, KKK192-194AAA-APC and 5A-APC (containing the combination of mutations present in the first two APC variants) given as a single bolus reduces 7-day mortality of mice given lethal doses of endotoxin. Administrations of a single bolus of 5A-APC after the initiation of sepsis also reduces mortality caused by LPS. 5A-APC with ≦8% of normal anticoagulant activity (which has reduced risk of bleeding) reduces mortality when given as two bolus administrations at 3 hours and then at 10 hours after initiation of bacterial infection, i.e. after onset of sepsis. This shows, first, that one or more bolus injections of APC or of APC variants, especially 5A-APC, can reduce mortality when given beginning hours after the onset of sepsis and, second, that it is not necessary to administer APC as a continuous infusion which is the current standard of practice because one or more bolus administrations can reduce mortality. Furthermore, dosages of approximately 0.06 to 0.4 mg / kg of APC and APC variants are identified to be sufficient to reduce mortality in sepsis.
Owner:VERSITI BLOOD RES INST FOUND INC +1
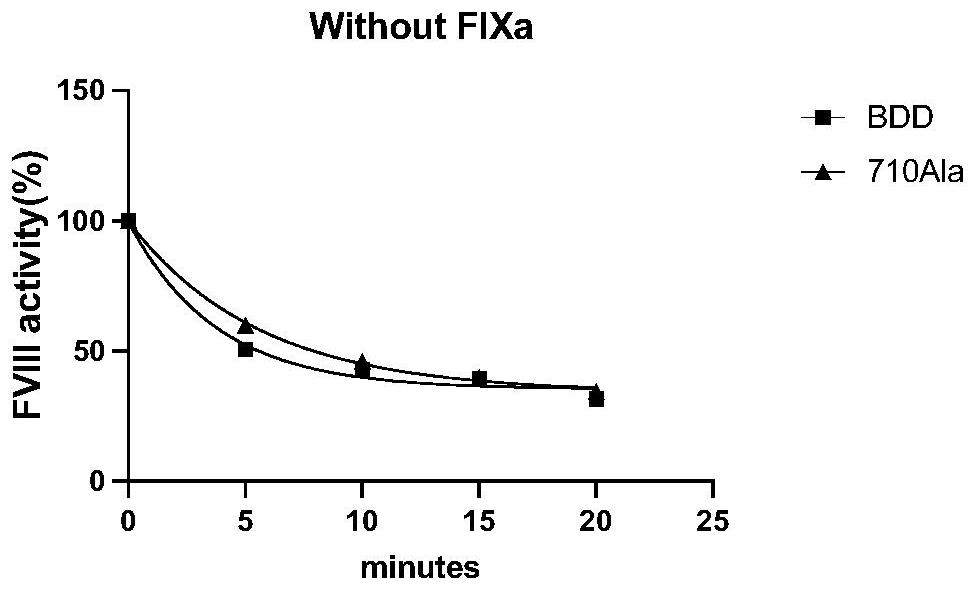
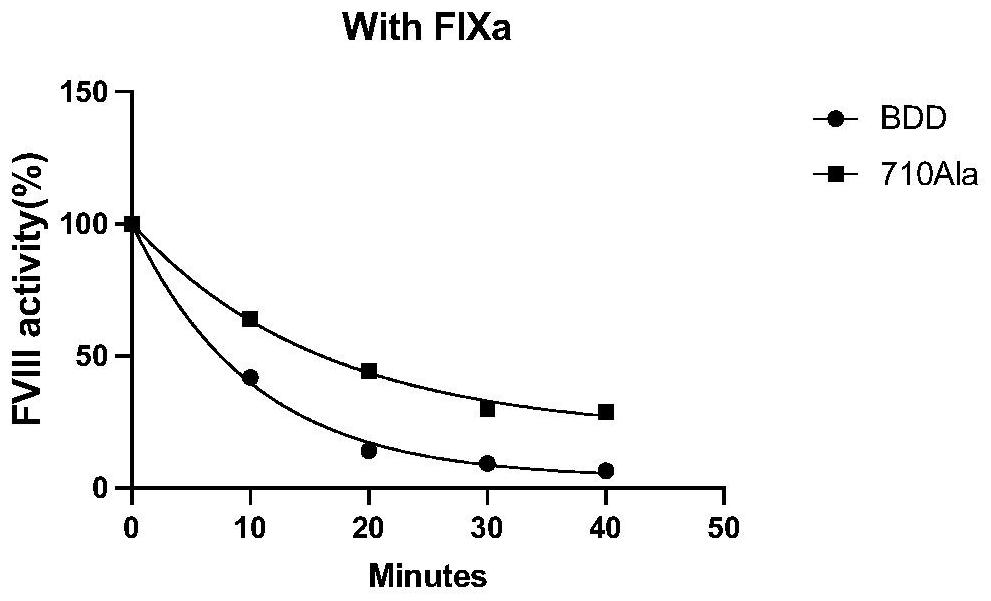
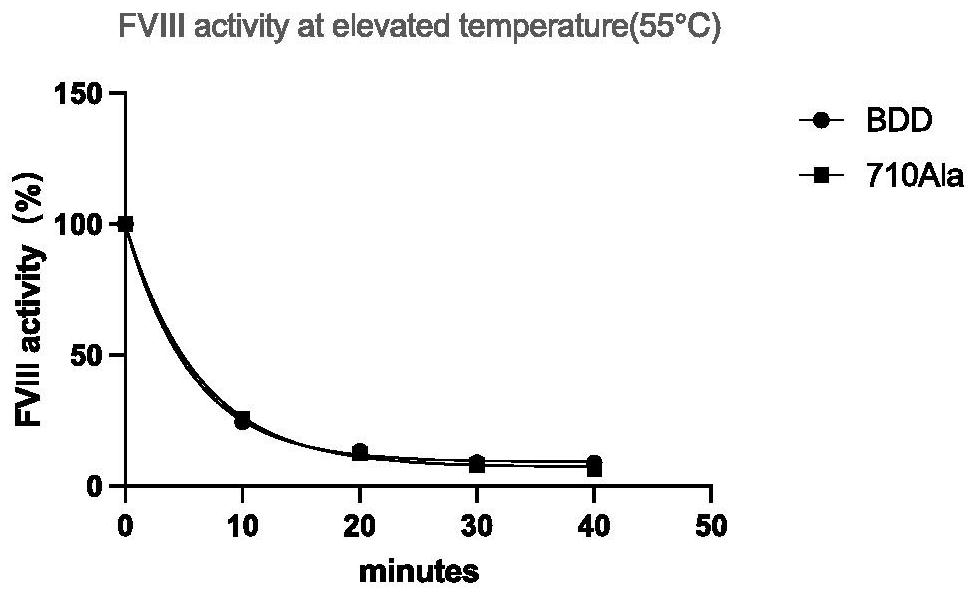
High-activity blood coagulation factor VIII or VIII polypeptide variant Gly710Ala
PendingCN114196677ADoes not destroy or inhibit coagulation activityDestroy or inhibit coagulation activityFactor VIIPeptide/protein ingredientsBlood coagulation factor VIIIEfficacy
The invention relates to a polypeptide variant Gly710Ala of a blood coagulation factor VIII or VIII a with high activity. The mutant does not destroy or inhibit the blood coagulation activity of FVIII, the blood coagulation specific activity is 1.5-2 times that of a wild type, and the mutant is more excellent in stability; the mutant obviously improves the drug efficacy of the FVIII, and has a good clinical application prospect.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGXI MEDICAL UNIV
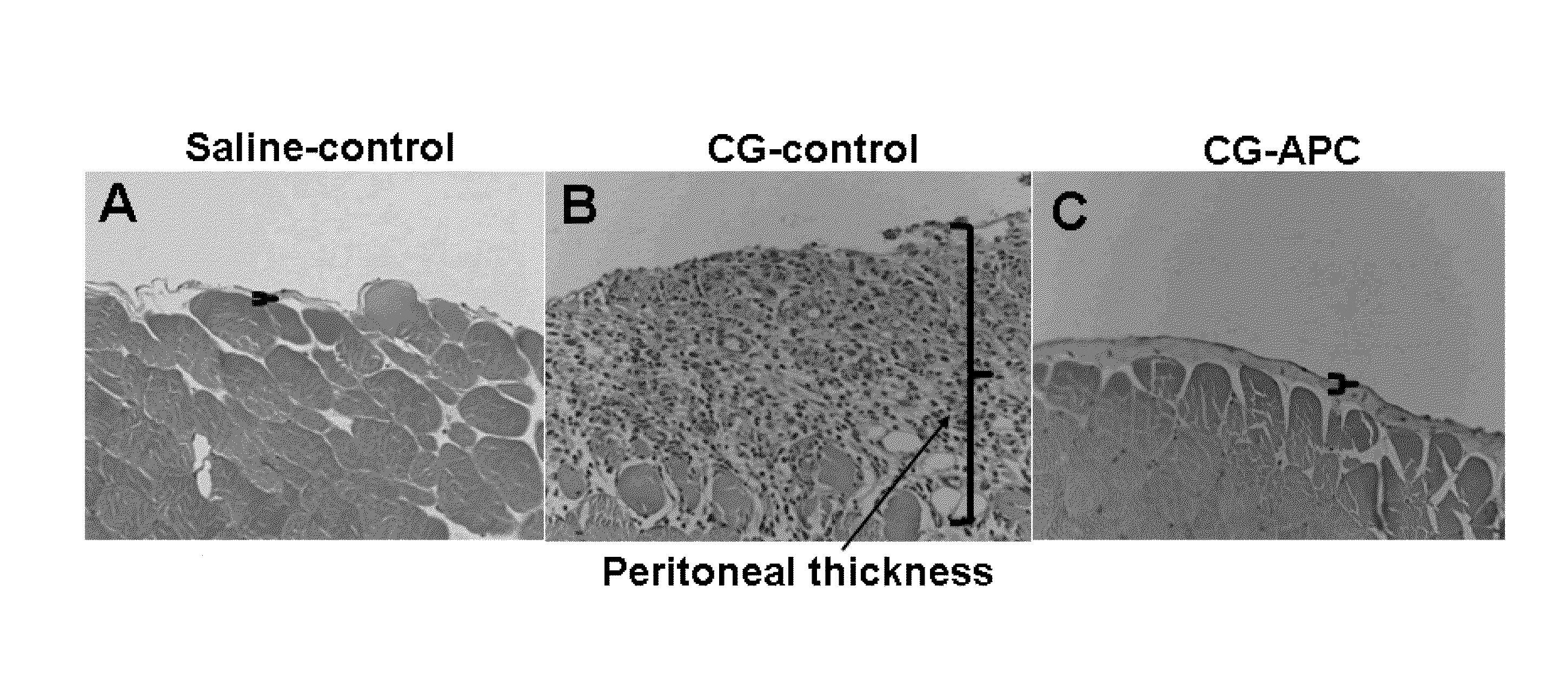
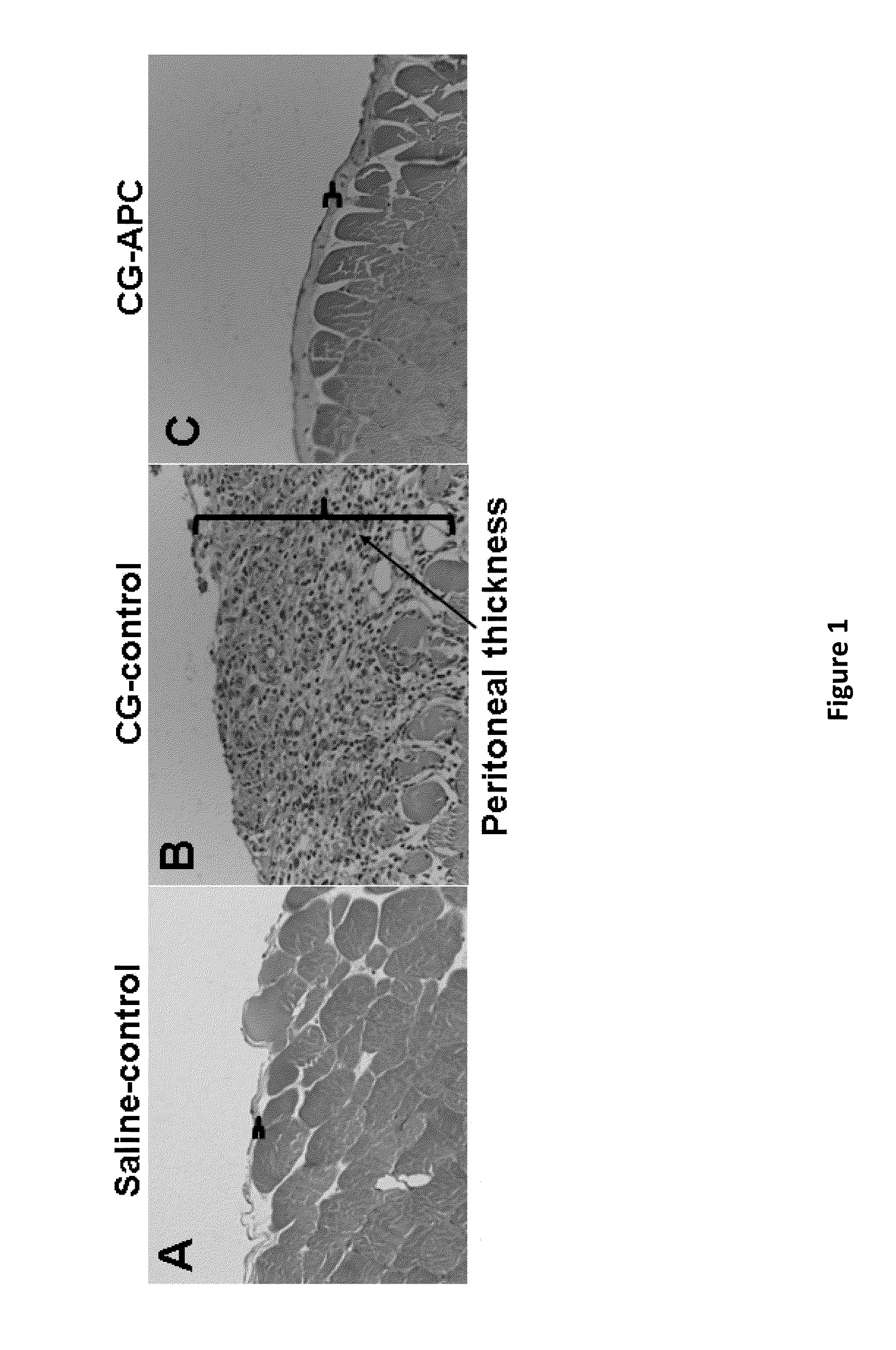
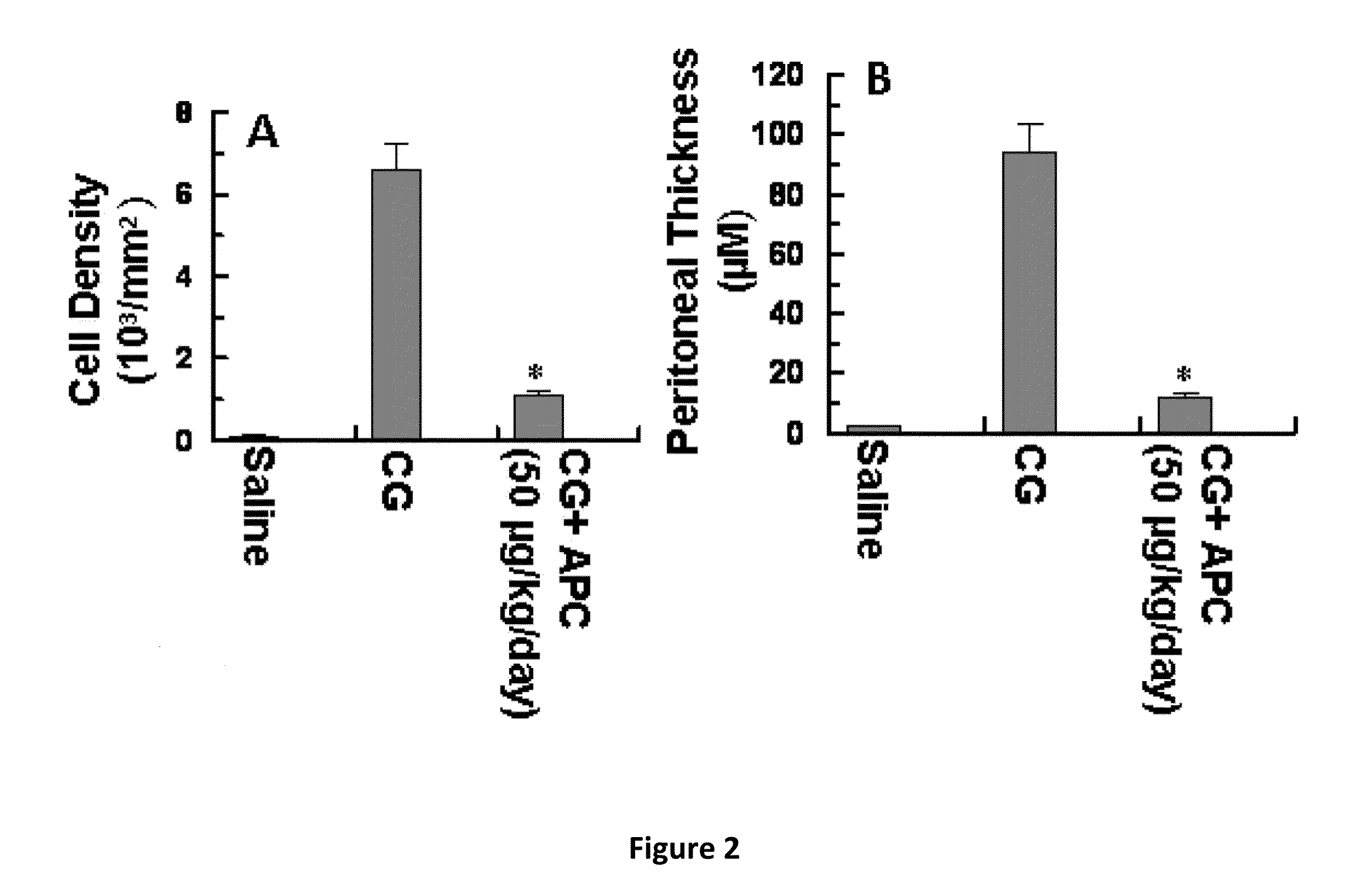
Methods for reducing fibrosis induced by peritoneal dialysis
InactiveUS20150366952A1Reduce morbidityPeptide/protein ingredientsSkeletal disorderProtein activationWild type
The disclosure relates to a method of preventing, inhibiting, or reducing fibrosis, the incidence of fibrosis or the progression of fibrosis associated with peritoneal dialysis, during or after peritoneal is administered. More specifically, the methods relates to using intraperitoneal administration of activated protein C (APC) possessing cytoprotective or anti-inflammatory activity, to reduce the incidence or progression of fibrosis associated with peritoneal dialysis. The method is demonstrated using wild type APC and a mutant APC possessing cytoprotective or anti-inflammatory activity but lacking anti-coagulant activity.
Owner:SAINT LOUIS UNIVERSITY
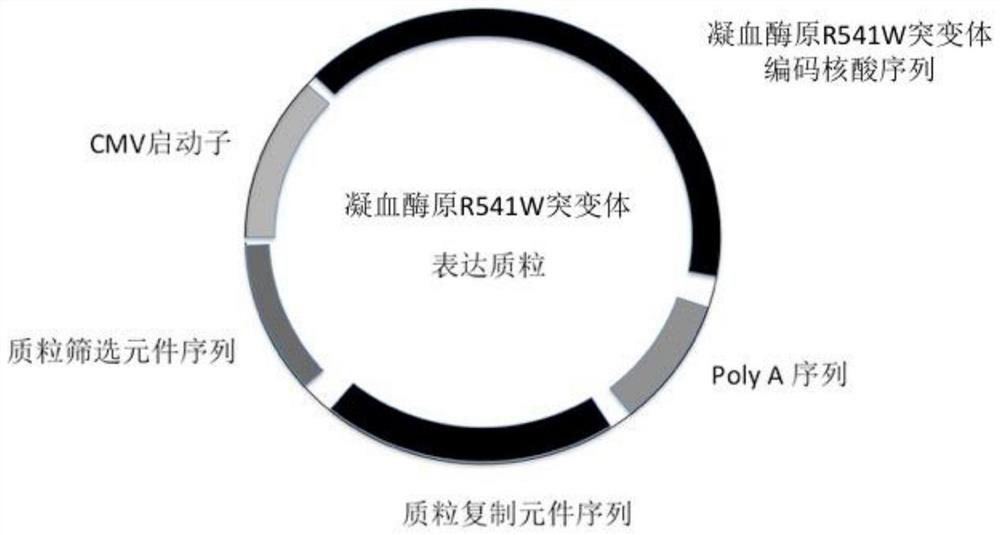
Application of a kind of prothrombin mutant protein and its coding nucleic acid
ActiveCN109260462BSelective procoagulant activityImprove conveniencePeptide/protein ingredientsBlood disorderMutated proteinPharmaceutical drug
The invention relates to the application of a prothrombin mutant protein and its coding nucleic acid for preparing gene therapy medicine, including linking it with a promoter and / or termination sequence and constructing an expression plasmid. The mutant protein and its encoding nucleic acid of the present invention have high blood coagulation activity, can efficiently promote blood coagulation, improve the overall blood coagulation function of the body, and have good prospects for gene therapy, gene editing and recombinant protein replacement therapy.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE +3
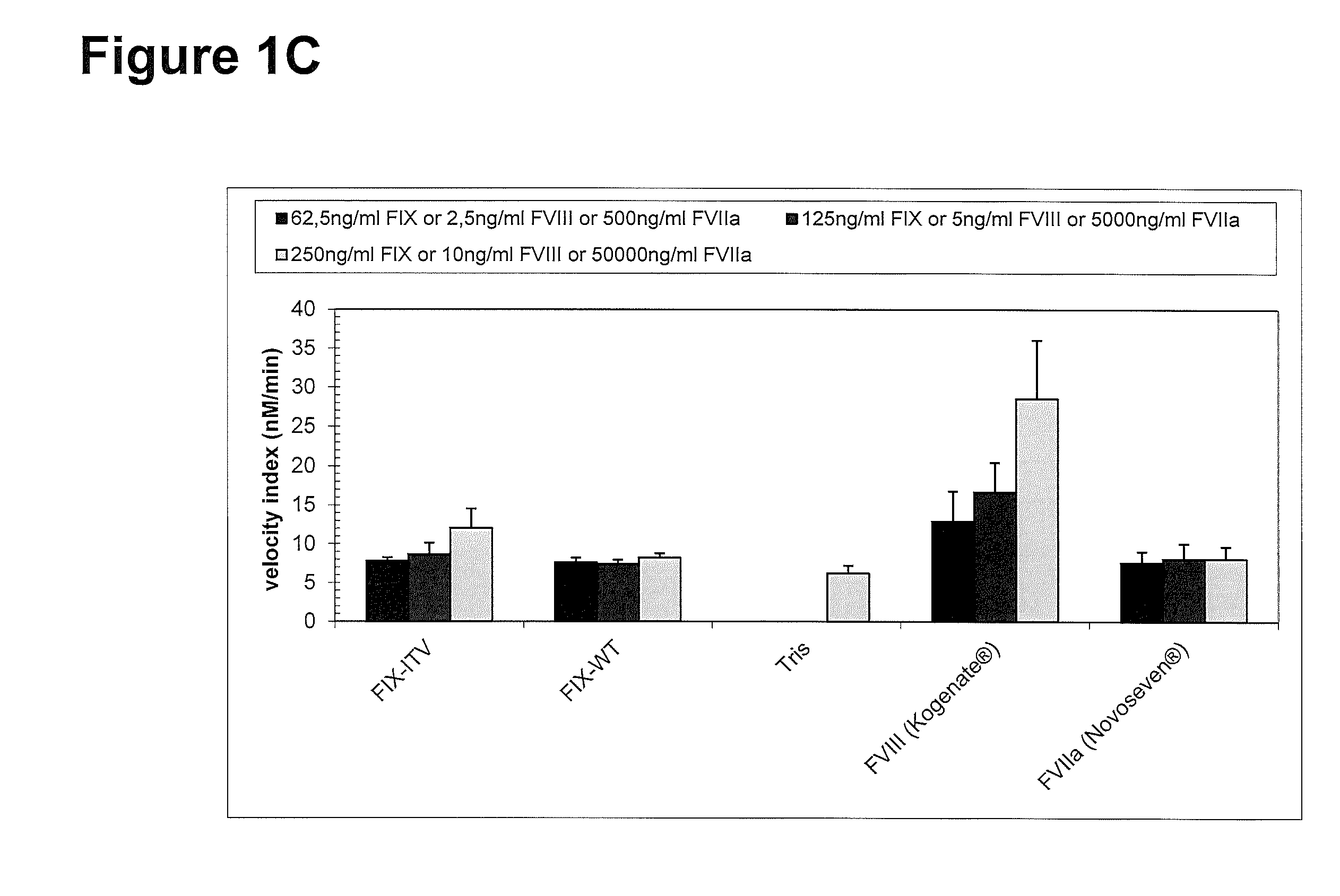
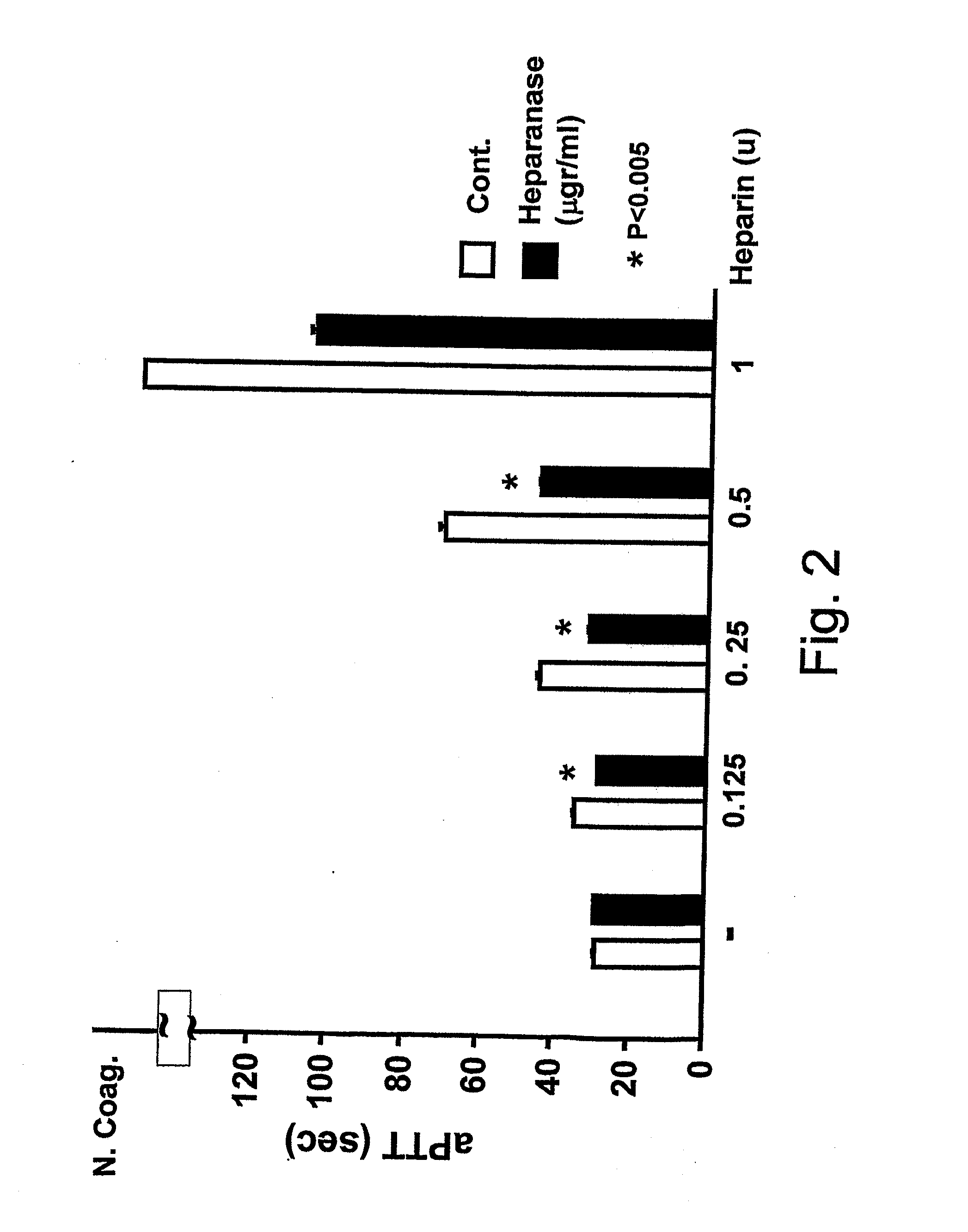
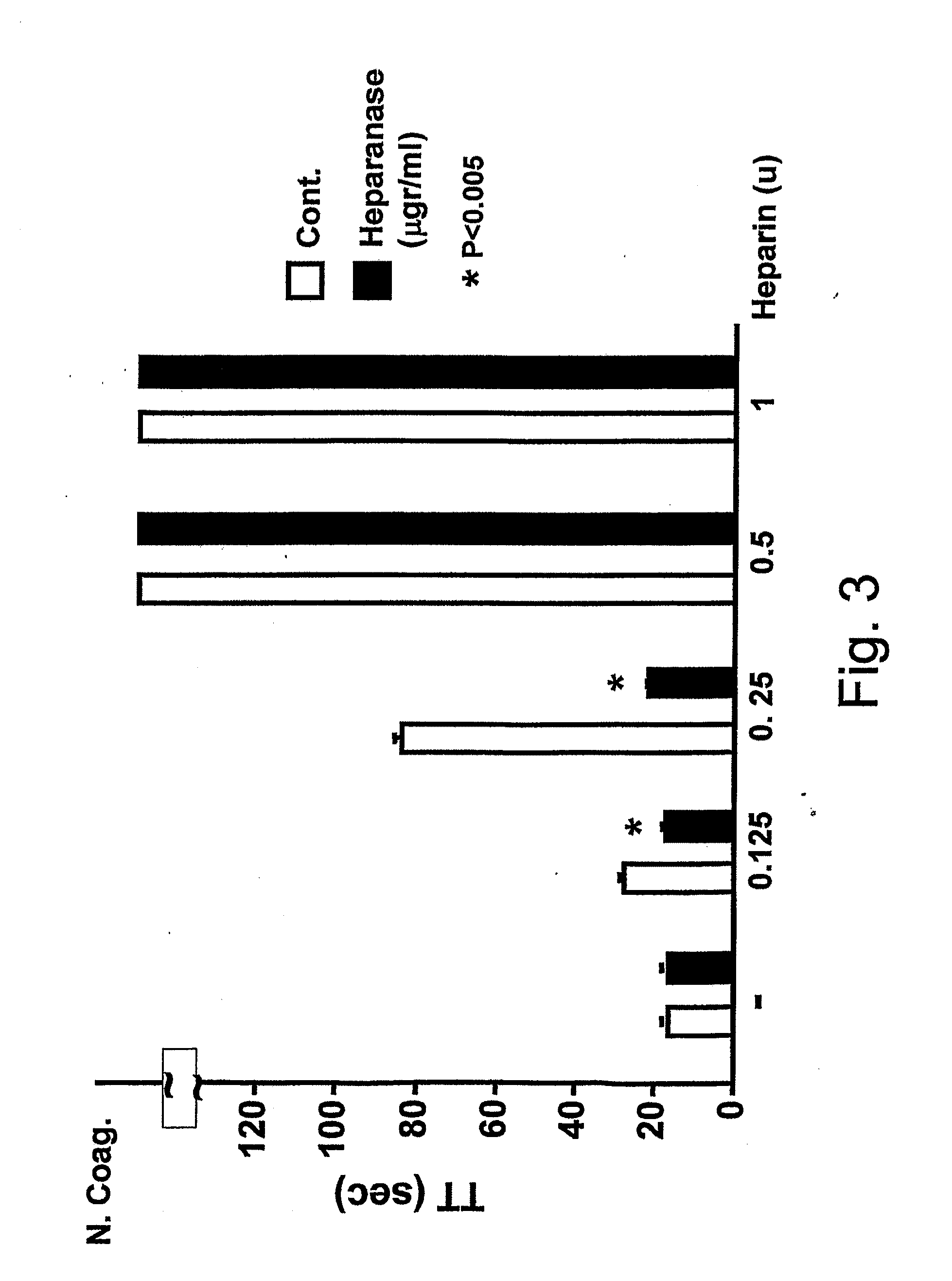
Methods and kits for assessing heparanase procoagulant activity, compositions comprising heparanase, and methods for the treatment of coagulation-related disorders
ActiveUS20190219601A1Candidate for therapeutic useInhibition is effectiveOrganic active ingredientsPeptide/protein ingredientsDiseaseTissue factor
The present invention relates to an inhibitory peptide capable of disrupting a Heparanase / Tissue Factor complex. The invention further provides methods and kits for determining heparanase procoagulant activity in a biological sample. The invention also relates to diagnostic methods for the detection and / or monitoring of a coagulation-related pathologic disorder in a mammalian subject. The invention further relates to compositions comprising heparanase and at least one tissue factor, uses and methods based thereon in the treatment, amelioration and prevention of coagulation-related pathologic conditions. The invention still further relates to methods for screening a coagulation modulatory compound, methods and uses based thereon in the treatment, amelioration and prevention of coagulation-related pathologic conditions.
Owner:RAMBAM MED TECH
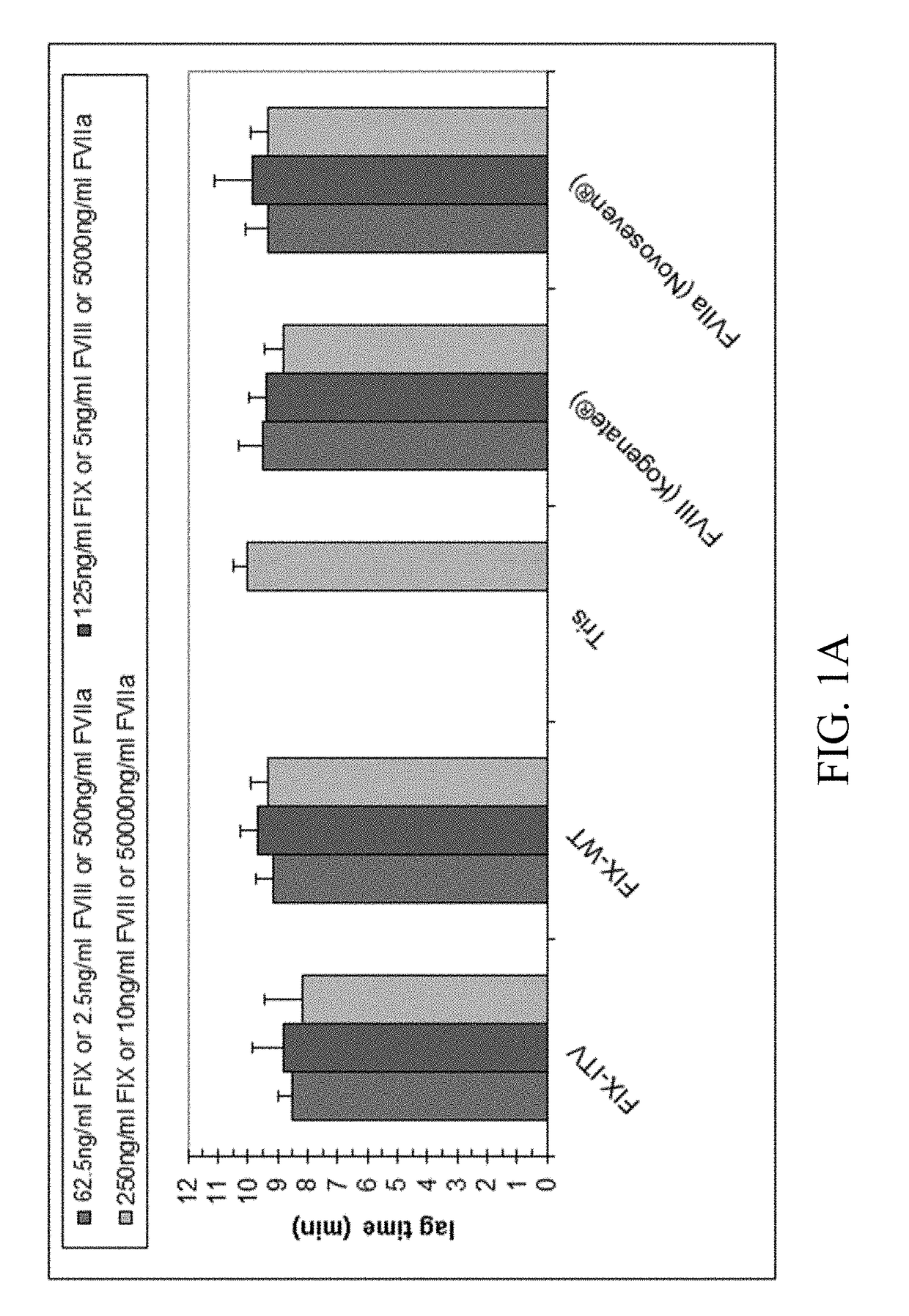
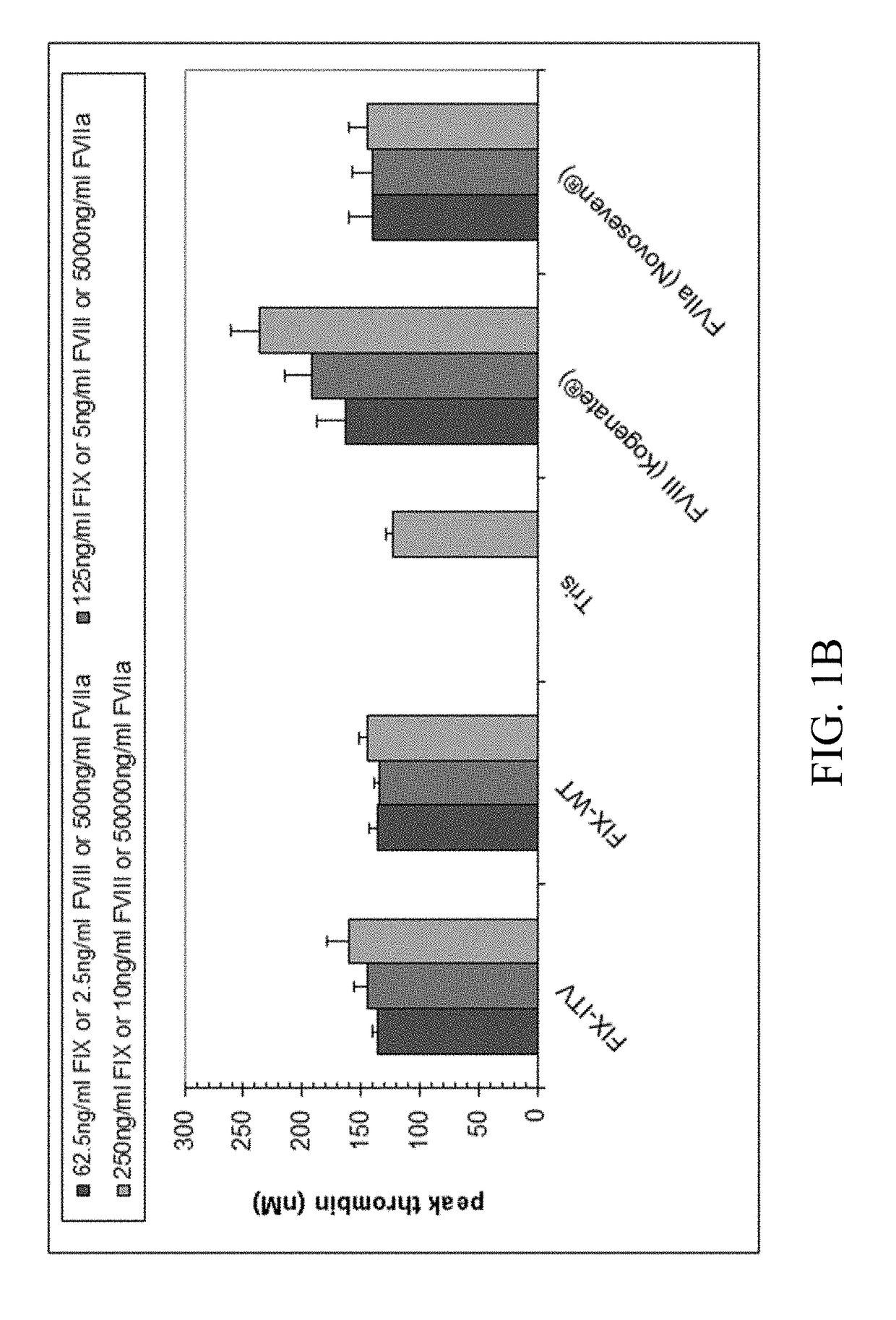
Medicinal composition usable for preventing and/or treating blood coagulation factor ix abnormality, comprising multispecific antigen binding molecule replacing function of blood coagulation factor VIII
PendingCN110461358AImmunoglobulins against blood coagulation factorsHybrid immunoglobulinsBlood coagulation factor VIIIVon willebrand
The present inventors verified the coagulation promoting effect of multispecific antigen binding molecules, said molecules replacing the function of FVIII, using blood and plasma collected from patients with FIX abnormality. As a result, it is clarified that a multispecific antigen binding molecule replacing the function of FVIII is not only usable in a method for preventing and / or treating bleeding in hemophilia A, acquired hemophilia A, von Willebrand's disease and hemophilia C caused by the dysfunction of FVIII but also usable in a method for preventing and / or treating bleeding in FIX abnormality owing to the coagulation promoting activity thereof. It is also clarified that the effect of a FIX preparation can be enhanced by the combined use of the FIX preparation with the multispecificantigen binding molecule replacing the function of FVIII and this combined use appears promising as a combination therapy showing a stable hemostatic effect.
Owner:NARA MEDICAL UNIVERSITY +1
Amino acid-substituted coagulation factor V
InactiveUS7666990B2Reduces blood clotting activityReduce generationHydrolasesPeptide/protein ingredientsWild typeIncreased Coagulation Activity
There is provided FV derivatives that reduce blood clotting activity, by reducing thrombin generation, when compared to wild-type FV. In particular, the FV of the present invention comprises single-point and multi-point mutations, encompassed by aspartic acid 79 to glutamic acid 119 of the wild type sequence (SEQ ID NO:2). The derivatives can be used to treat patient with conditions necessitating reduced clotting activity.
Owner:CANADIAN BLOOD SERVICES
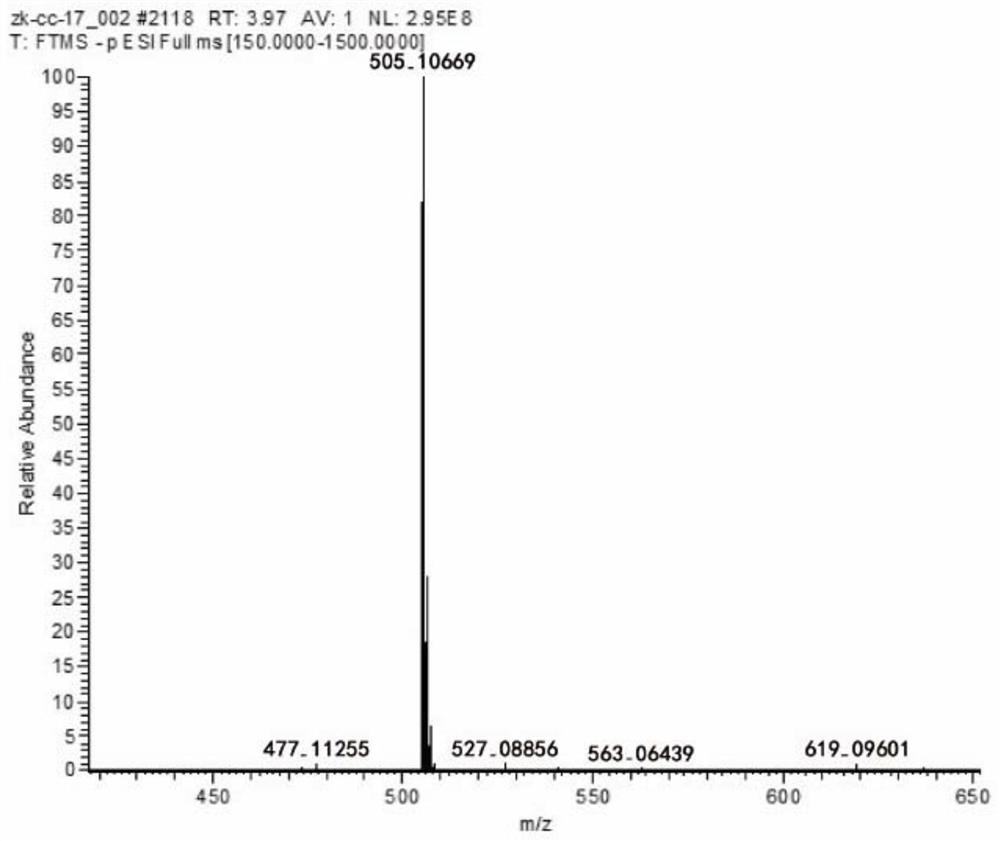
Use of a compound in medicine
ActiveCN110711189BGood water solubilityHigh purityOrganic active ingredientsOrganic chemistryAntithrombotic AgentMedical equipment
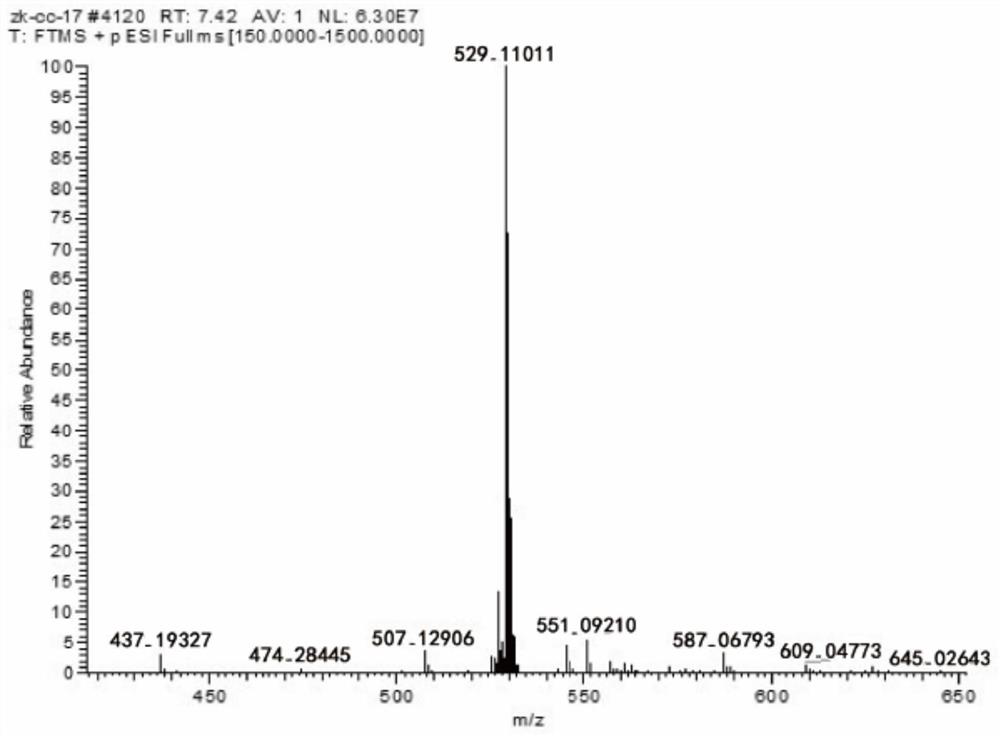
The invention discloses a compound, its preparation method and application. The molecular formula of the compound is C 27 H 22 O 10 , the molecular weight is 506. The compound obtained by the preparation method of the present invention has high purity, 98% purity, good stability and good biological activity. Through the method of in vitro coagulation activity test, it is found that the compound has a strong inhibitory activity on platelet aggregation activated by thrombin, and has the potential to develop anticoagulant or antithrombotic drugs and medical equipment.
Owner:FUJIAN SANAN SINO SCI PHOTOBIOTECH CO LTD
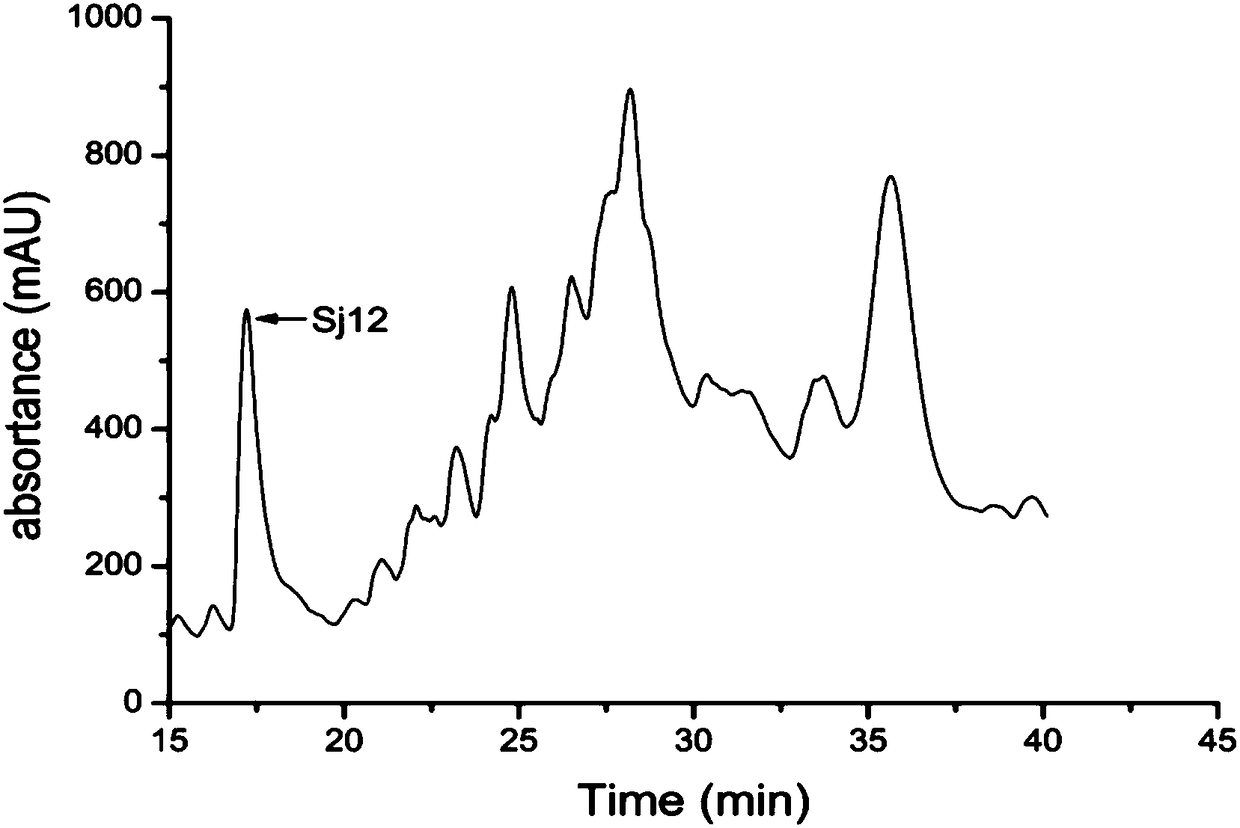
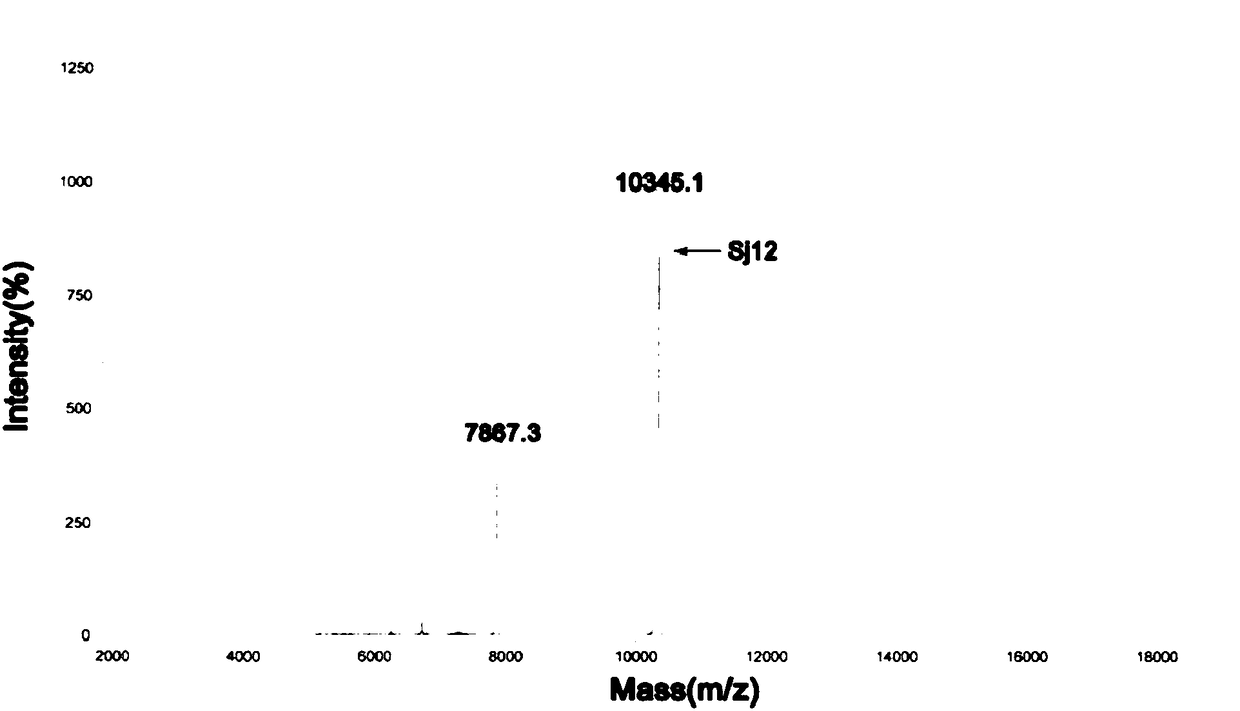
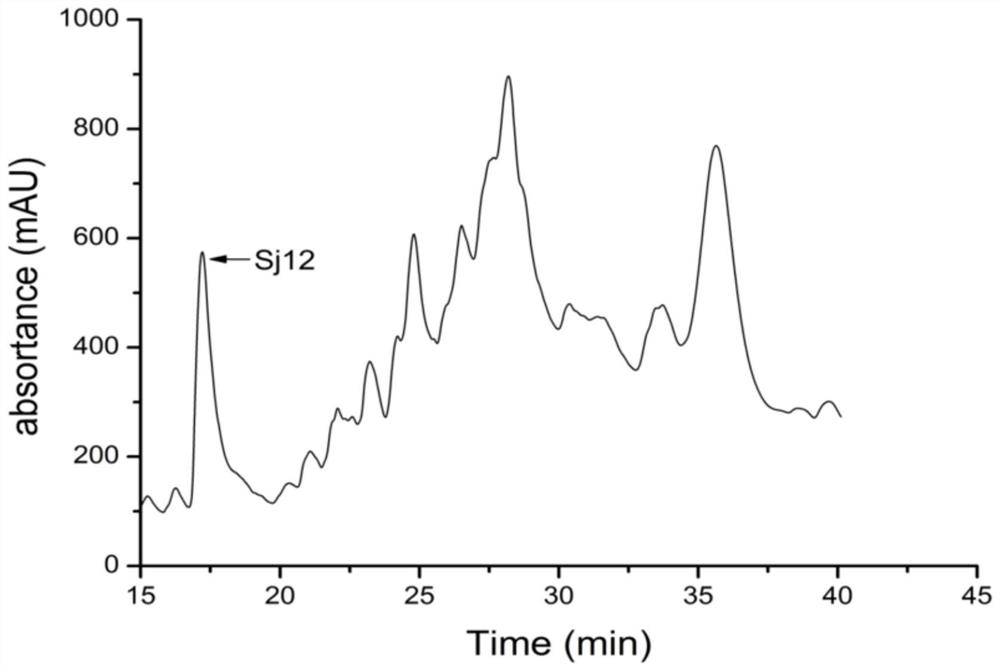
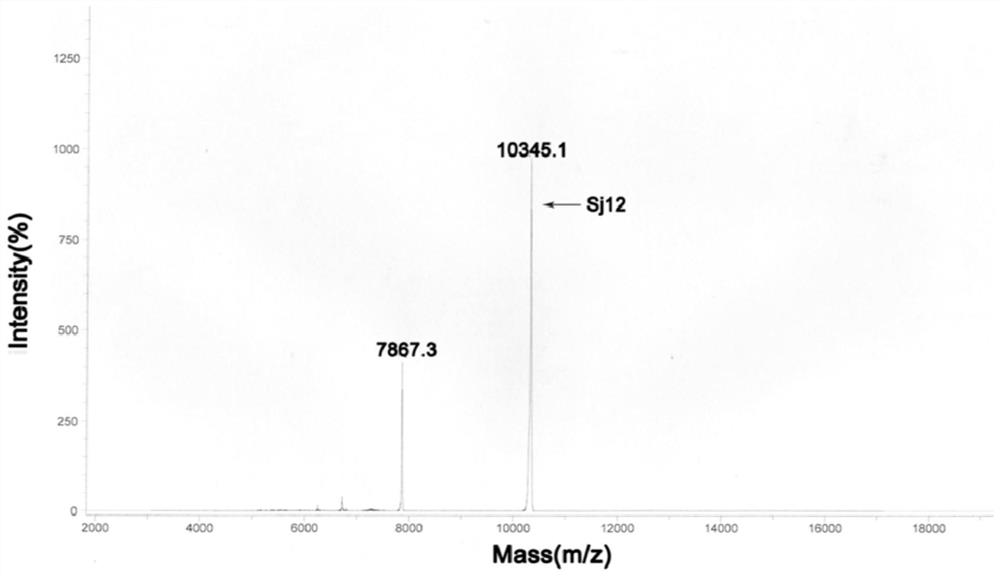
Sj12 polypeptide and application thereof in preparation of anticoagulation medicine
ActiveCN108314720APrevent coagulationHas development and application valuePeptide/protein ingredientsFermentationMedicineCoagulation system
The invention discloses an Sj12 polypeptide from schistosoma japonicum katsurada. The polypeptide has no influence on an extrinsic coagulation system and a coagulation common path, but can interfere an endogenic coagulation system, so that the coagulation time is prolonged to a value being 10 seconds or more longer than the normal detection range; the effect of inhibiting the blood coagulation isachieved; the coagulation activity is superior to that of anticoagulant peptide in the prior art; the important anticoagulant and antithrombotic medicine development and application values are realized.
Owner:HUBEI UNIVERSITY OF MEDICINE
Factor ix variants with clotting activity in absence of their cofactor and/or with increased f.ix clotting activity and their use for treating bleeding disorders
ActiveUS20180251744A1Correct hemophilic phenotypeSpecific activityCompound screeningApoptosis detectionActivated factor IXWild type
The present invention relates to variants of factor IX (F.IX) or activated factor IX (F.IXa), wherein the variant is characterized in that it has clotting activity in absence of its cofactor. The present invention furthermore relates to variants of factor IX (F.IX) or activated factor IX (F.IXa), wherein the variant is characterized in that it has increased F.IX clotting activity compared to wildtype. The present invention furthermore relates to the use of these variants for the treatment and / or prophylaxis of bleeding disorders, in particular hemophilia A and / or hemophilia B or hemophilia caused or complicated by inhibitory antibodies to F.VIII. The present invention also relates to further variants of factor IX (F.IX) which have desired properties and can, thus be tailored for respective specific therapeutic applications.
Owner:DRK BLUTSPENDEDIENST BADEN WURTTEMBERG HESSEN GGMBH
Preparation and application of a highly active blood coagulation factor xi mutant and its gene therapy/editing vector, recombinant/fusion protein
ActiveCN108220274BImprove coagulation functionGood prospects for alternative treatmentsPeptide/protein ingredientsNucleic acid vectorDiseaseBlood coagulations
The present invention relates to the preparation and application of a highly active blood coagulation factor XI mutant and its gene therapy / editing vector, recombinant / fusion protein. The nucleotide sequence is such as SEQ ID NO: 1-6, and the amino acid sequence is such as SEQ ID NO: 7 shown. The invention has high blood coagulation activity, can efficiently activate blood coagulation reaction, improve the overall blood coagulation function of the body, is applied to the treatment of hemorrhagic diseases, and has good prospects for gene therapy, gene editing and recombinant protein replacement therapy.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE +3
Compositions of human prothrombin and activated factor X for improving hemostasis in the treatment of bleeding disorders
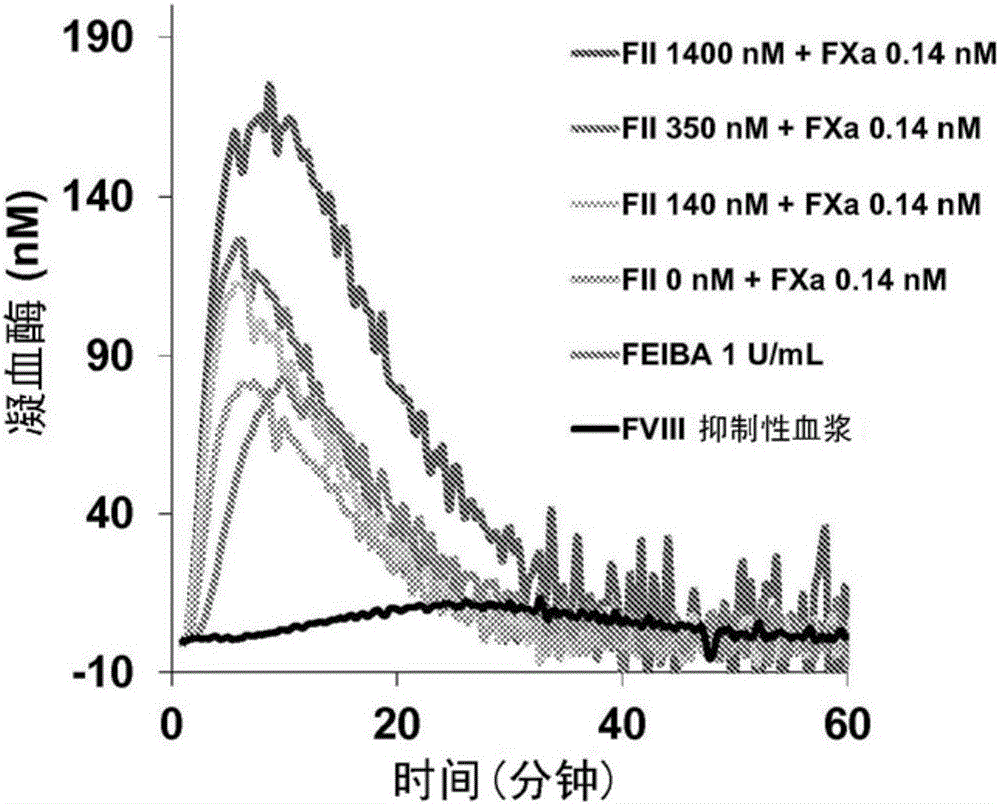
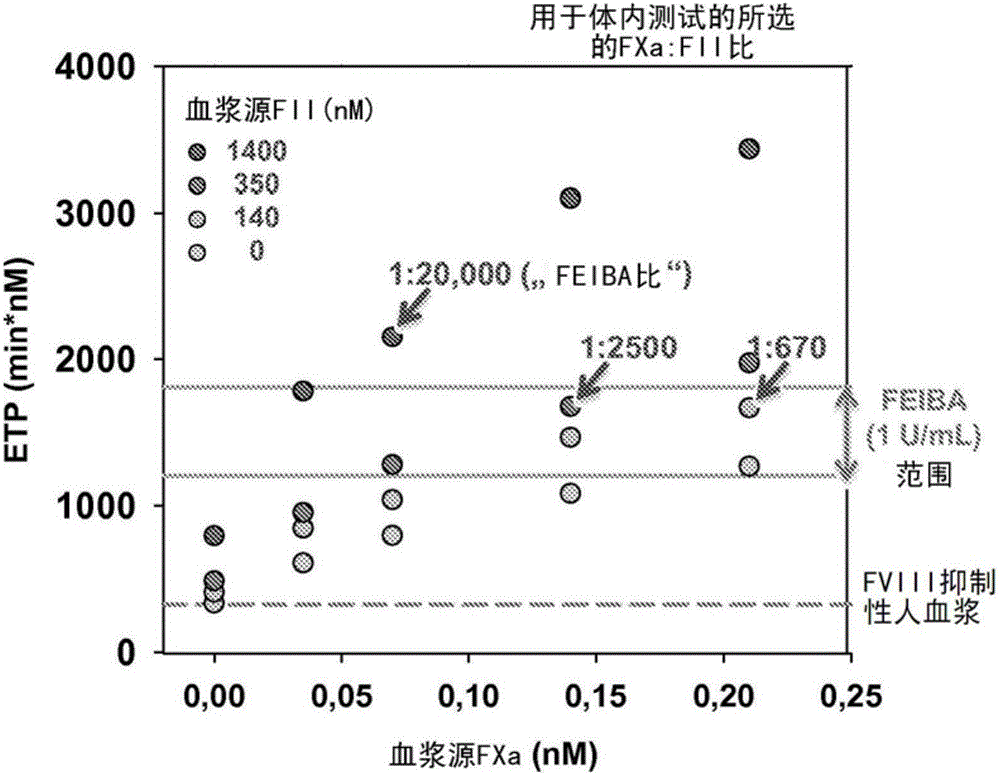
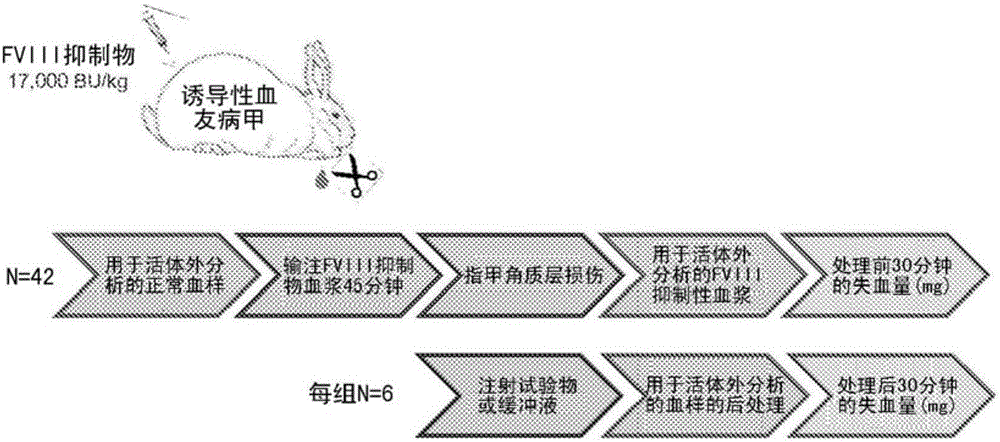
Disclosed herein are compositions and methods for improving hemostasis in the treatment of bleeding disorders and reversal of anticoagulant activity. Effective ratios of prothrombin (FII) and activated factor X (FXa) for the treatment of bleeding disorders that are as efficacious as FEIBA, but require a lower concentration of FII are described herein.
Owner:TAKEDA PHARMA CO LTD
Compositions, medicaments and sgRNAs for increasing coagulation activity in hemophilia b patients
ActiveCN108977464BPeptide/protein ingredientsStable introduction of DNAPharmaceutical drugB hemophilia
The invention discloses a composition for improving the coagulation activity of a patient with type B hemophilia, a medicine and sgRNA (small guide Ribonucleic Acid), and relates to the field of genetreatment of hemophilia. The composition comprises a first AAV (Adeno-associated Virus) vector and a second AAV vector for expressing the sgRNA; a core sequence of the sgRNA is selected from SEQ ID NO. 6 to SEQ ID NO. 9. The composition can be used for treating the type B hemophilia and recovering the coagulation activity.
Owner:成都金唯科生物科技有限公司
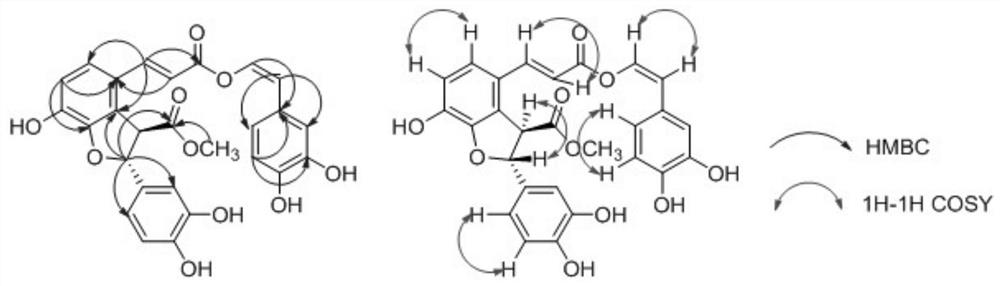
A kind of phenylpropanoid compound and preparation method thereof
ActiveCN110698444BGood water solubilityHigh purityOrganic active ingredientsOrganic chemistryAntithrombotic AgentMedical equipment
Owner:FUJIAN SANAN SINO SCI PHOTOBIOTECH CO LTD
sj12 polypeptide and its application in the preparation of anticoagulant drugs
ActiveCN108314720BPrevent coagulationHas development and application valuePeptide/protein ingredientsFermentationPharmaceutical drugAnticoagulant drug
Owner:HUBEI UNIVERSITY OF MEDICINE
A highly active coagulation factor xi mutant ala570thr
ActiveCN112126636BImprove catalytic abilityHigh catalytic activityPeptide/protein ingredientsGenetic material ingredientsZymogenDisease
The present invention relates to a highly active coagulation factor XI mutant Ala570Thr (A570T), the nucleotide sequence is shown in SEQ ID NO: 1-4, and the amino acid sequence is shown in SEQ ID NO: 5. After being activated from the zymogen state to become an active enzyme, the present invention is resistant to its physiological inhibitor, so it has high blood coagulation activity and stronger catalytic ability to non-physiological substrates, and is applied to the treatment of hemorrhagic diseases , has good prospects for gene therapy, gene editing and recombinant protein replacement therapy.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com