Coagulation factor binding proteins and uses thereof
A blood coagulation factor and binding protein technology, applied in the direction of anticoagulation factor immunoglobulin, coagulation/fibrinolytic factor, VII factor, etc., can solve the problem of prolonging and short half-life of blood coagulation factors, and achieve good safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0874] Example 1: Generation and Purification of Recombinant Antibodies
[0875] Expression constructs are generated using standard molecular biology methods. Nucleotides encoding the antibody, Annexin A5 (NP_001145; SEQ ID NO: 14) and GS linker (SEQ ID NO: 20) were synthesized by Geneart (Thermo Fisher Scientific, NY, USA) sequence. The sequences were amplified by PCR, digested by restriction enzymes and cloned into expression vectors by T4 DNA ligase.
[0876] Antibodies were generated according to Table 1 below.
[0877] Table 1: Recombinant Antibodies
[0878]
[0879]
[0880] Recombinant plasmid DNA was purified using the QIAprep Spin Miniprep kit (QIAGEN, Hilden, Germany) and quantified by Nanodrop UV spectrophotometer. Confirm the sequence before transfection.
[0881] According to the manufacturer's instructions, use the Expi293F TM Expression Systems All transfections were performed by transient transfection.
[0882] Proteins were harvested from the res...
Embodiment 2
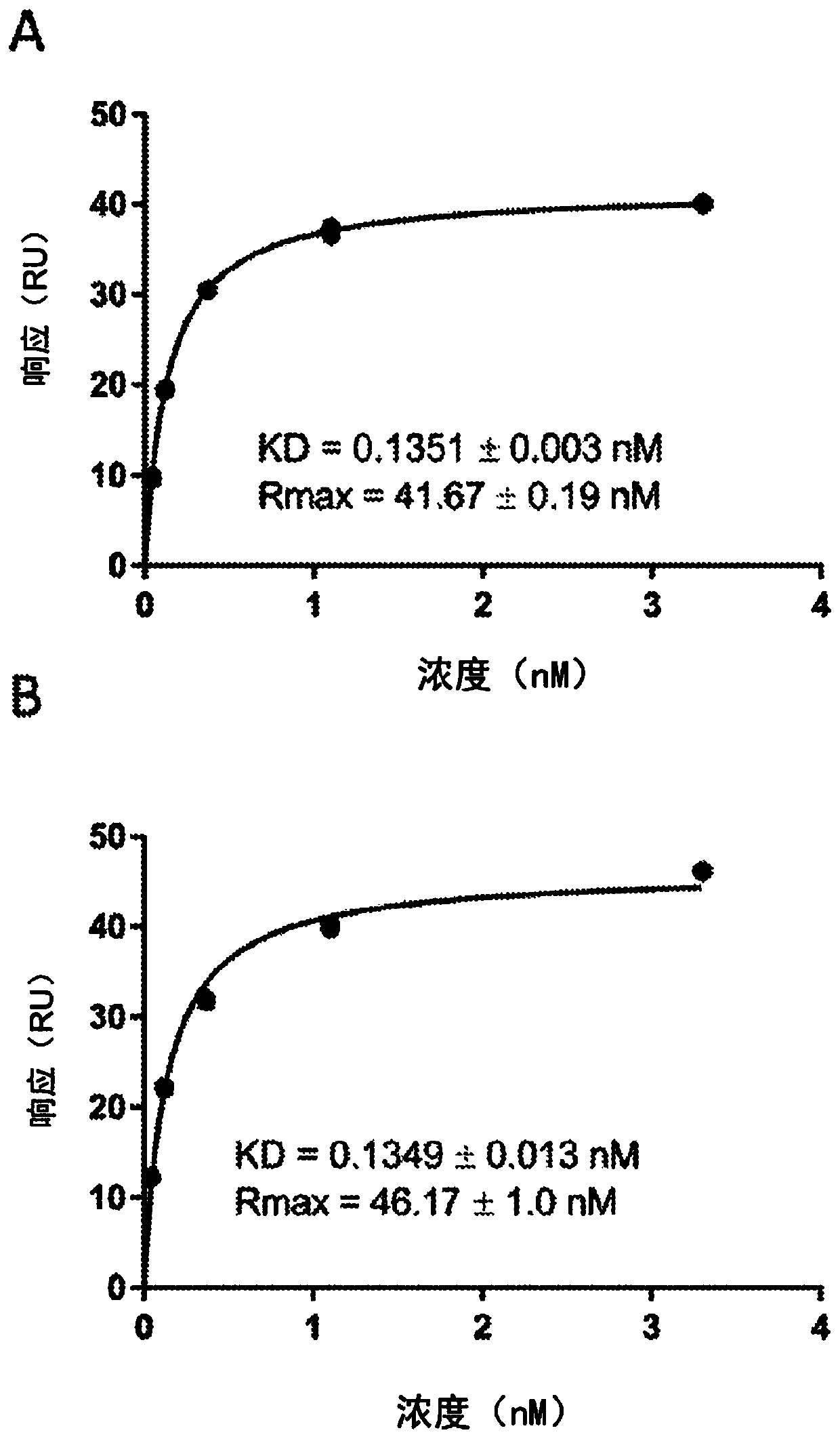
[0883] Example 2: Annexin A5-linked antibodies are membrane-targeted
[0884] To assess whether the Annexin A5-linked antibody targets the cell membrane, a biosensor analysis of the Annexin A5-linked antibody was performed. Briefly, a phosphatidylserine (PS) / phosphatidylcholine (PC) / phosphatidylethanolamine (PE) (PS / PC / PE-biotinyl 70:25:5) phospholipid mixture was dissolved in TRIS [20mM] pH 8.0, NaCl [150 mM], NOG [2 mM], and vesicles were obtained using sonication. Phospholipids lacking phosphatidylserine (PC / PE-biotinyl 95:5) were prepared in a similar manner as reference surfaces in biosensor studies.
[0885] Phosphatidylserine-containing vesicles were immobilized at low levels on an SA sensor chip-docked On the active flow cell of the T-200 biosensor. Vesicles lacking PS were immobilized on upstream reference cells. Binding to PS / PC / PE was assessed by injecting 3.3, 1.1, 0.37, 0.12 and 0.04 nM aFIX-Annexin A5 (CSL4060) for 5 minutes at 37°C. The data were fitted to...
Embodiment 3
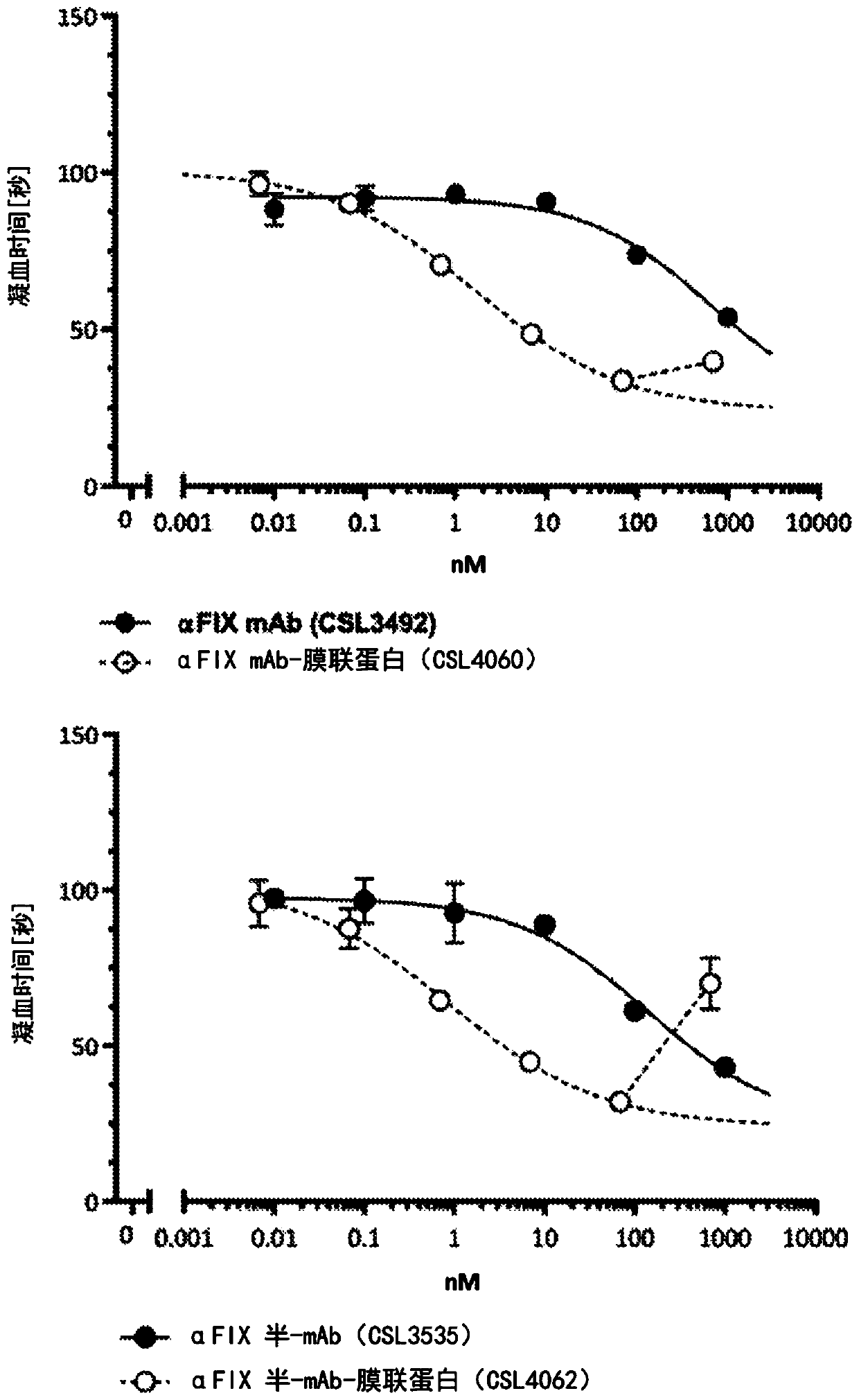
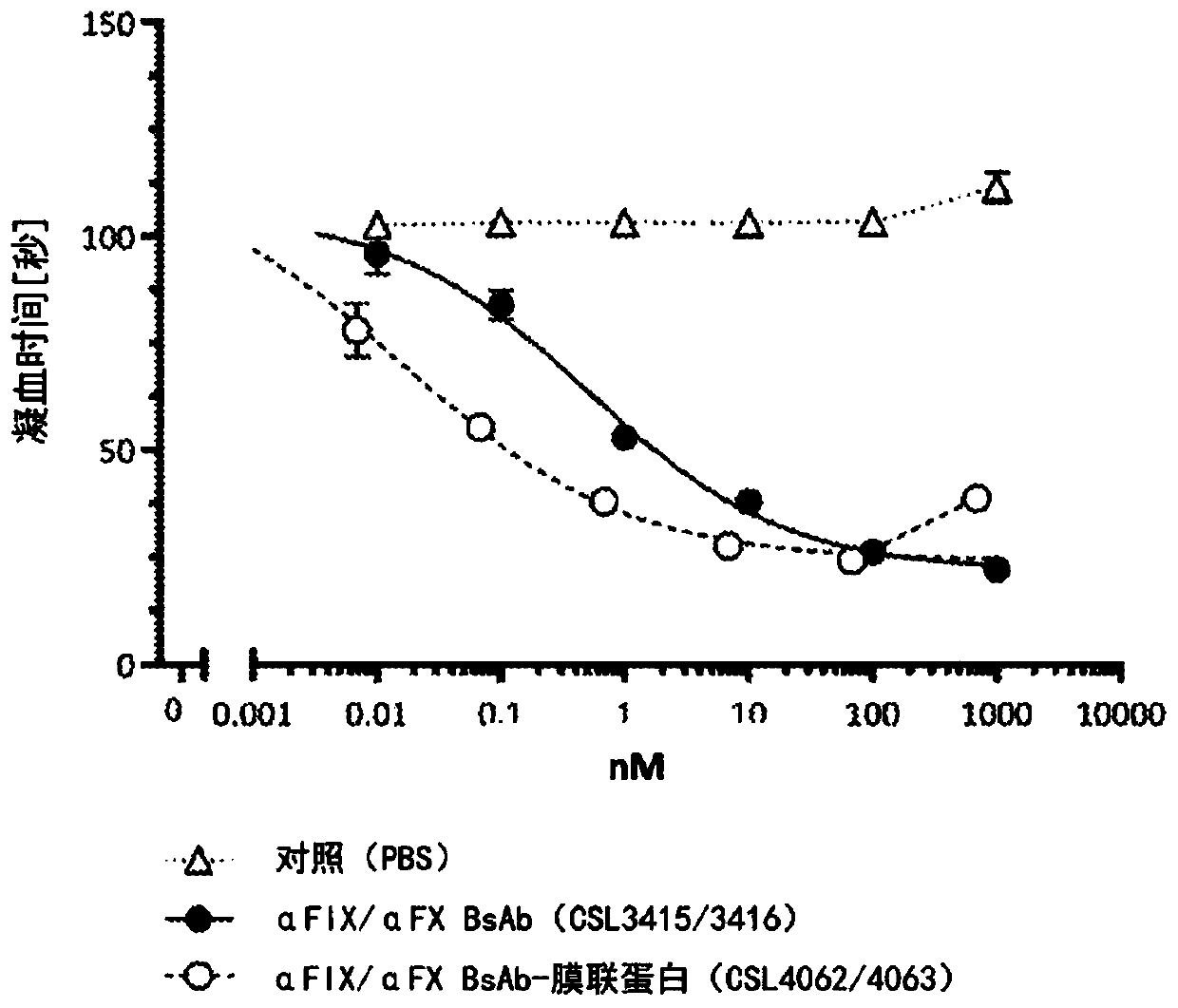
[0887] Example 3: Membrane Targeting Anti-Factor IX Monospecific Whole and Half Antibodies Have Enhanced Factor VII Bypassing Activity Compared to Non-Targeting Antibodies
[0888] In order to study the potential factor VIII-bypass activity and coagulation activity of the generated antibodies, the Thrombolyzer Compact X system (Behnk Elektronik, Inc.) with standard assay reagents from Siemens Healthcare (Siemens, Germany) was used according to the manufacturer's instructions. Germany) measures the activated partial thromboplastin time (aPTT). Antibodies were diluted in FVIII deficient plasma (Siemens Healthcare) as indicated to achieve final concentrations ranging from 1000 nM to 1 pM. Briefly, 50 μl of each dilution was mixed with 50 μl of aPTT reagent (Pathromtin SL) on one side of a Thrombolyzer cuvette. 50ul of CaCl 2 [25mM] was added to the other side of the Thrombolyzer cuvette and allowed to equilibrate to 37°C. by adding CaCl 2 The solution was mixed with an antibo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com