Preparation and application of a highly active blood coagulation factor xi mutant and its gene therapy/editing vector, recombinant/fusion protein
一种凝血因子、突变蛋白的技术,应用在基因治疗、肽/蛋白质成分、载体等方向,能够解决限制、效率低下等问题,达到强催化能力、增强凝血活性、高凝血活性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
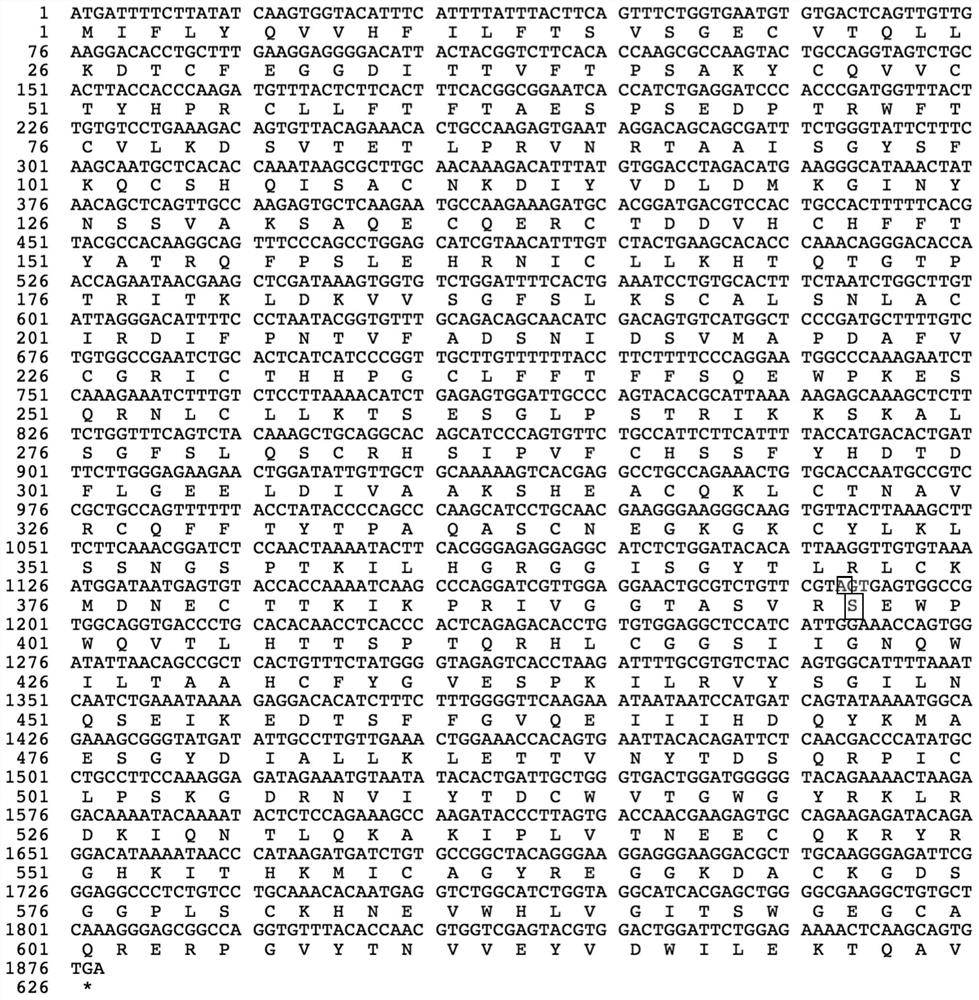
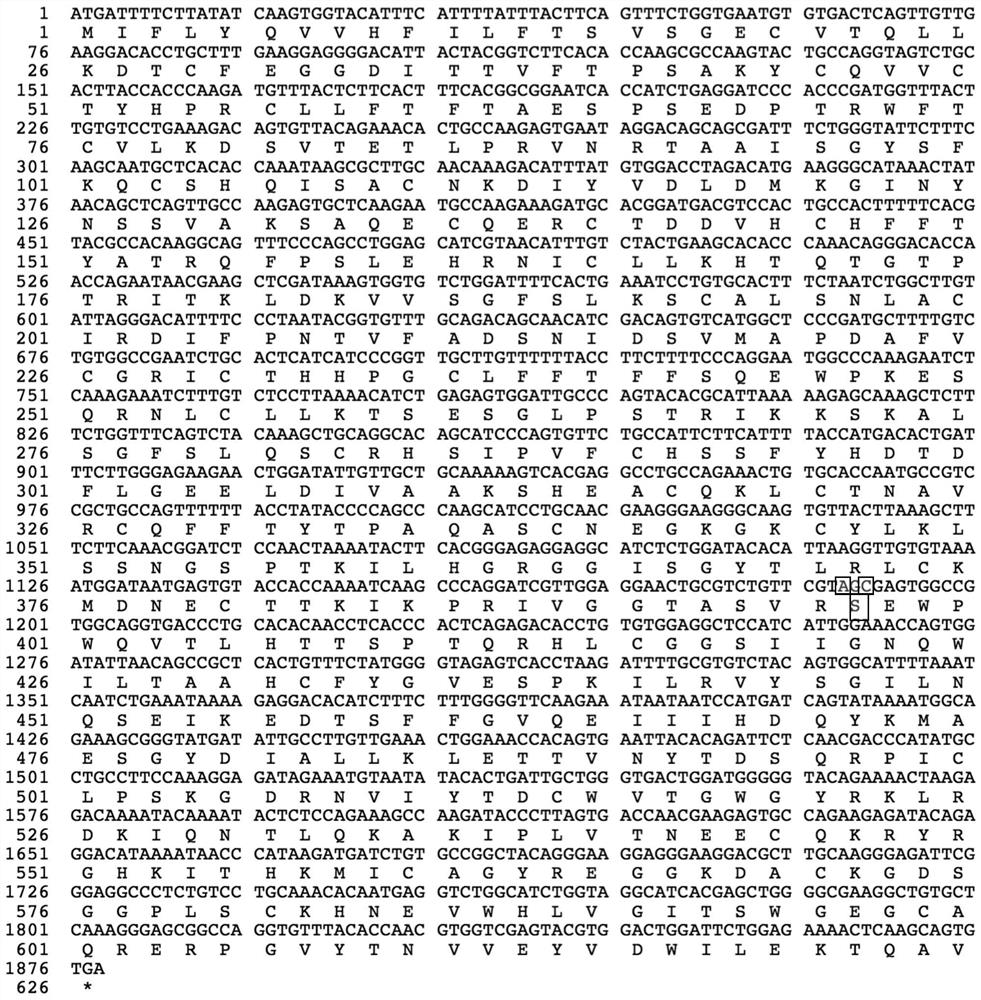
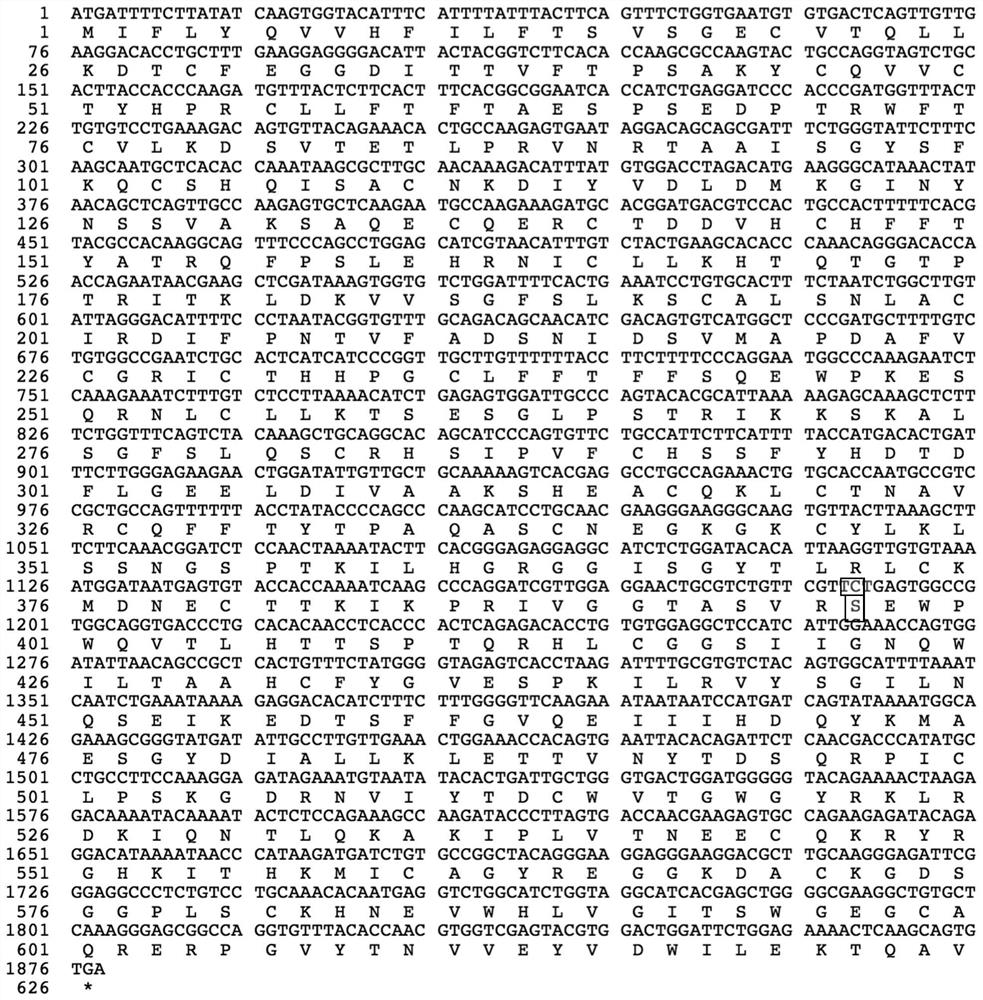
[0046] The amino acid sequence of the mutant protein of highly active blood coagulation factor XI is shown in SEQ ID NO:7.
[0047] A method for preparing a mutant protein of highly active blood coagulation factor XI, comprising the steps of:
[0048] (1) The human blood coagulation factor XI coding gene of human wild type or blood coagulation factor XI mutant Gly397Ser is connected in the vector to obtain the recombinant vector; (see Figure 8 )
[0049] (2) Transforming the above-mentioned recombinant vector into a host cell to obtain recombinant expression cell clones;
[0050] (3) Cultivate the above-mentioned cell clones in a serum-free medium to express the expression of a mutant protein of highly active blood coagulation factor XI;
[0051] The serum-free medium is "SAFC Biosciences EX-CELL TM 302” (commercialized reagent). In order to ensure product safety and prevent blood-derived preparations from spreading infectious diseases, serum-free medium is used for mamma...
Embodiment 2
[0064] The preparation method of the gene therapy plasmid vector of highly active blood coagulation factor XI comprises the following steps:
[0065] (1) Construction of the vector plasmid The cDNA encoding FXI Gly397Ser was ligated into the pcDNA3.1 plasmid containing the CMV promoter.
[0066] (2) Inject the purified vector into type A hemophilia mice. Hemophilia mice aged 4-8 weeks were selected, and 150 μg of the plasmid vector dissolved in 2 mL of normal saline was injected into 6-7 mice respectively through the tail vein. Six mice were injected with PBS as negative control.
[0067] (3) aPTT detects coagulation factor XI activity. 48 hours after injection, orbital blood was collected to detect coagulation factor XI activity and antigen, wherein the activity was the sum of the original mouse coagulation factor XI activity and the activity of injected human wild-type or mutant coagulation factor XI in the mouse, while the antigenic principle was only is the level of pou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com