Oral medicament for treating cerebral infarction and relieving limb spasm
A technology for cerebral infarction and limbs, applied in the field of plant materials, can solve the problems of non-concentrated curative effect, unprominent curative effect, and weak pertinence, etc., and achieve the effects of reducing infarct size, improving hemorheology indicators, and prolonging gasping time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
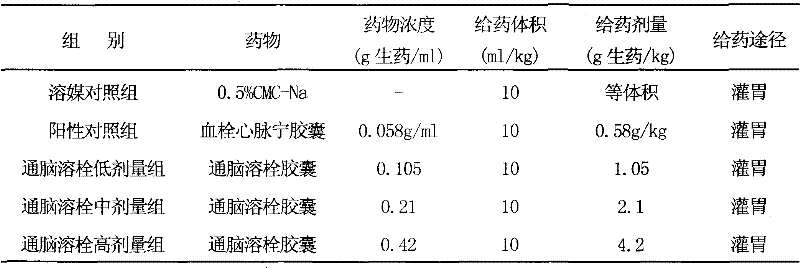
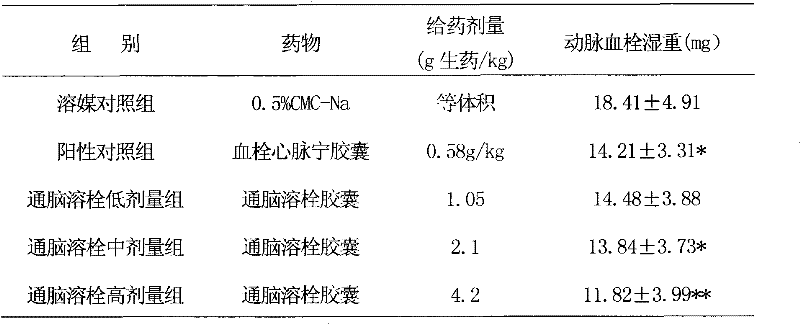
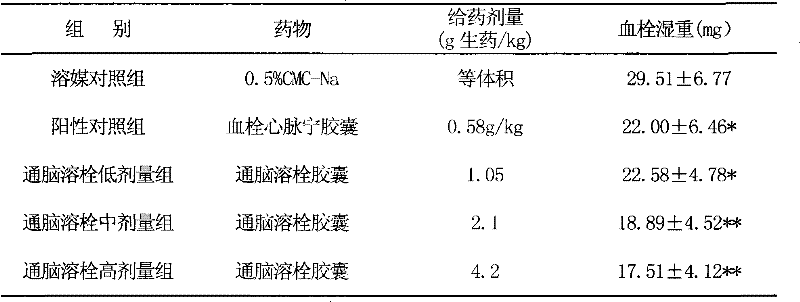
Image
Examples
Embodiment 1
[0040] Taking the production of 1000 pharmaceutical capsule products of the present invention as an example, the used Chinese medicine raw materials and auxiliary materials and their proportioning ratio are:
[0041] Astragalus 270g
[0042] Pueraria 270g
[0043] Radix Paeoniae Alba 210g
[0044] Chuanxiong 180g
[0045] 150g peach kernels
[0046] Milletweed 180g
[0047] Tendon Grass 180g
[0048] Wexgrass 180g
[0049] Achyranthes knuckle 120g
[0050] Dilong 60g
[0051] Add dextrin to 400g
[0052] Its preparation method is as follows:
[0053] Take astragalus and other ten kinds of herbs, 90g of kudzu herbs, powder, pass through a 100-mesh sieve, and set aside; the remaining kudzu herbs and other nine herbs are decocted twice, adding 10 times the amount of water for 2 hours for the first time. Add 8 times the amount of water twice and decoct for 2 hours, filter, combine the filtrates, concentrate to a relative density of 1.20±0.05 (measured at 60°C), add ethan...
Embodiment 2
[0089] Taking the production of 1000 pharmaceutical capsule products of the present invention as an example, the used Chinese medicine raw materials and auxiliary materials and their proportioning ratio are:
[0090] Astragalus 231g
[0091] Pueraria 231g
[0092] Radix Paeoniae Rubra 194g
[0093] Chuanxiong 194g
[0094] 194g peach kernels
[0095] Milletweed 194g
[0096] Tendon Grass 194g
[0097] Wexgrass 194g
[0098] Achyranthes knuckle 116g
[0099] Dilong 58g
[0100] Add dextrin to 400g
[0101] Its preparation process is identical with the preparation process of embodiment 1 capsule. Each grain weighs 0.4g, and each gram contains 4.5g of traditional Chinese medicine raw materials.
[0102] Taking the production of 1000 tablets of the pharmaceutical tablet product of the present invention as an example, the used Chinese medicine raw materials and auxiliary materials and their proportioning ratio are as follows:
[0103] The raw materials used in the tablet...
Embodiment 3
[0137] Taking the production of 1000 pharmaceutical capsule products of the present invention as an example, the used Chinese medicine raw materials and auxiliary materials and their proportioning ratio are:
[0138] Astragalus 282g
[0139] Pueraria 282g
[0140] Radix Paeoniae Alba 176g
[0141] Chuanxiong 176g
[0142] 141g peach kernels
[0143] Spatholobus 177g
[0144] Tendon Grass 177g
[0145] Wexgrass 177g
[0146] Achyranthes knuckle 141g
[0147] Dilong 71g
[0148] Add dextrin to 400g
[0149] Its preparation process is identical with the preparation process of embodiment 1 capsule. Each grain weighs 0.4g, and each gram contains 4.5g of traditional Chinese medicine raw materials.
[0150] Taking the production of 1000 tablets of the pharmaceutical tablet product of the present invention as an example, the used Chinese medicine raw materials and auxiliary materials and their proportioning ratio are:
[0151] The raw materials used in the tablet of this em...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com