A highly active coagulation factor xi mutant ala570thr
A blood coagulation factor, high activity technology, applied in the direction of blood diseases, genetic engineering, plant gene improvement, etc., can solve the problems of limitation and low efficiency, achieve strong catalytic ability, enhance blood coagulation activity, and good prospects for alternative treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
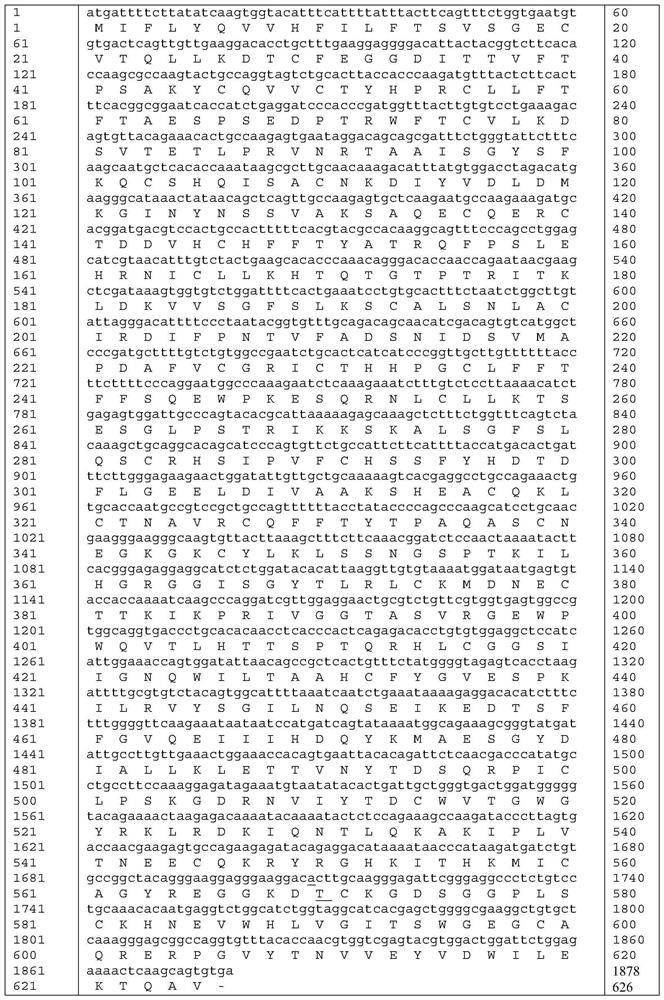
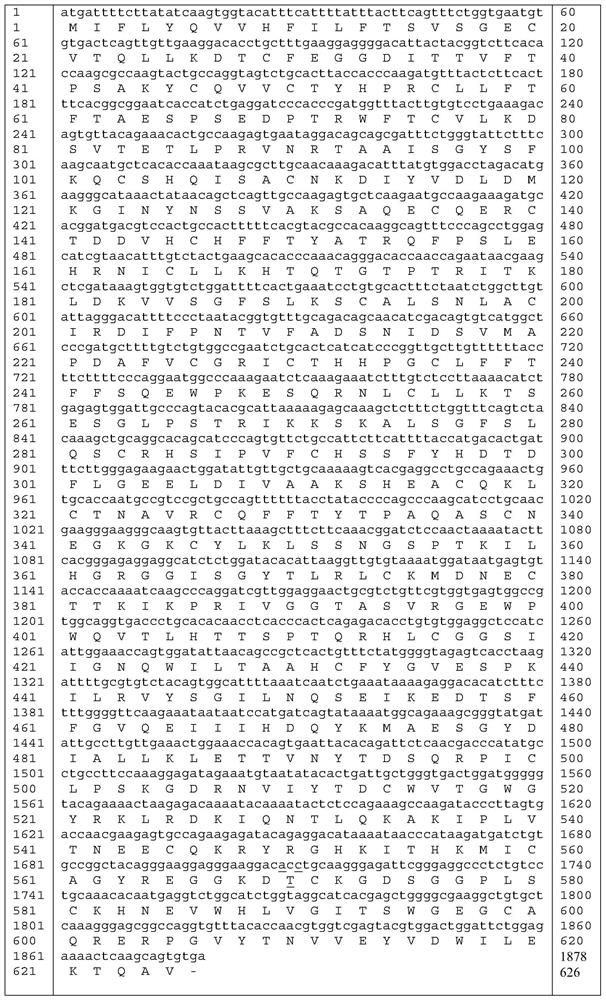
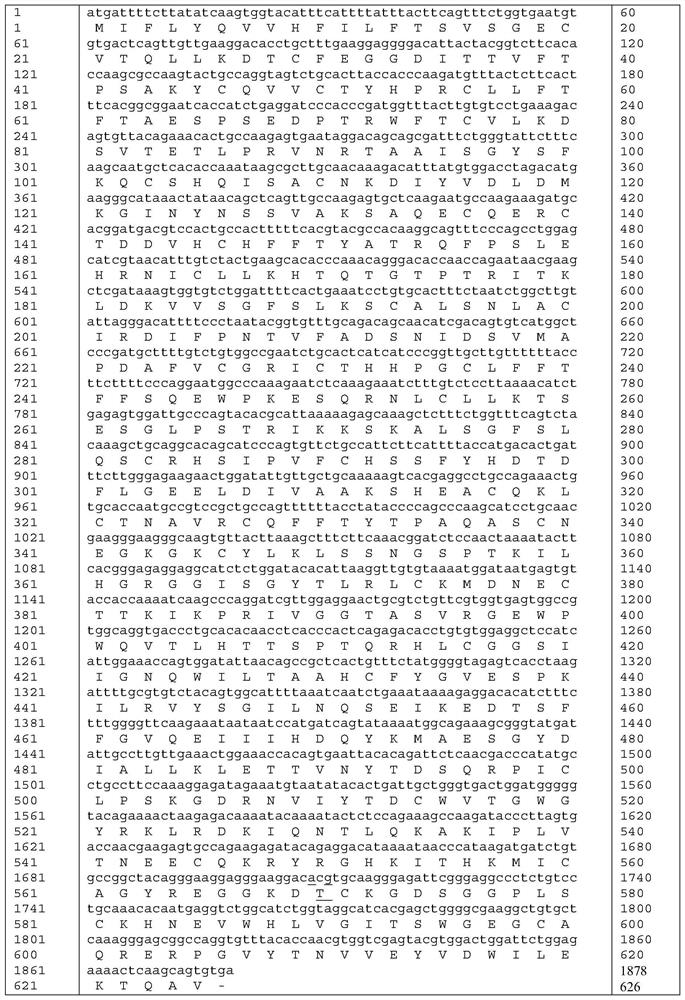
[0036] The amino acid sequence of mutant proteins of highly active coagulation factor XI mutant ALA570THR is shown in SEQ ID NO: 5.
[0037] Method for preparing mutant proteins of highly active coagulation factor XI mutant ALA570THR, including the following steps:
[0038](1) Costling factor XI encoding genes of human wild-shaped or coagulation factor XI mutant Ala570 THR is entered into the vector, resulting in a recombinant carrier; Image 6 )
[0039] (2) Transform the above recombinant carrier to obtain recombinant expression cytocytes;
[0040] (3) Culture of the above cell clones in serum-free medium, expressing the expression of macrosurbs of highly active coagulation factor XI mutant ALA570THR;
[0041] Serum-free medium for "SAFC Biosciences EX-Cell TM 302 "(commercial reagents). To ensure product safety, prevent blood source preparations from propagating infectious diseases, so serum-free medium is used for mammalian cell culture, protein expression, and cellular vectors...
Embodiment 2
[0054] Detect thrombus force map (see Figure 9)
[0055] ThrombolaStogram (TEG): It is a comprehensive test for monitoring all blood coagulation processes in whole blood. It does not require blood specimens, with a small number of whole blood monitoring of coagulation factors, platelets, fibrinogen, fibrin, fibrin, and its cellular components, accurately providing patient coagulation profiles. The anticoagulation blood is added to the activation monitoring reagent bottle first, and then the volume is added to the special cylindrical cup (add CaCl in advance. 2 . The cup is rotated at an angle of 4 ° 45 'and 1 weeks / 9S, and a needle is monitored by a spirally suspended needle in the blood, and the coagulation speed and intensity curve is drawn by a computer. The coagulation process is mainly evaluated by the following curve parameters: (1) The reaction time R value, that is, the detection start to the curve amplitude to rise to 2 mm, refers to the time required to detect the fibr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com