Method for improving high-efficiency expression of recombinant human coagulation factor VIII
A human coagulation factor, high-efficiency expression technology, applied in the field of genetic engineering, can solve the problems of exogenous DNA overload, difficult to meet industrialization requirements, complex cell line construction, etc., and achieve stable and high-efficiency expression and secretion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Construction of recombinant gene Azu-rhFVIII
[0030] 1. Total gene synthesis of optimized sequence SP and recombinant human coagulation factor VIII (SP-rhFVIII)
[0031] According to literature (L.Thim, B.Vandahl, J.Karlsson.et al(2010). and characterization of a new recombinant factor VIII (N8).Haemopbilia.16,349-359) published the original signal peptide SP (nucleotide sequence is SEQ ID NO: 2) and the amino acid sequence of rhFVIII (total 1464aa), according to Chinese hamster ovum ( CHO) optimization principle, the nucleic acid sequence of SP and rhFVIII is optimized. At the same time, the enzyme cutting sites in the sequence are strictly limited. The optimized sequence cannot contain HindIII, EcoRI, SalI, NotI, and PvuI enzyme cutting sites. At the same time, HindIII and Kozak sequences are added to the 5' end of the sequence, and the A double stop codon and EcoRI were added to the end to obtain the optimized SP (nucleotide sequence is SEQ ID NO: 3), a...
Embodiment 2
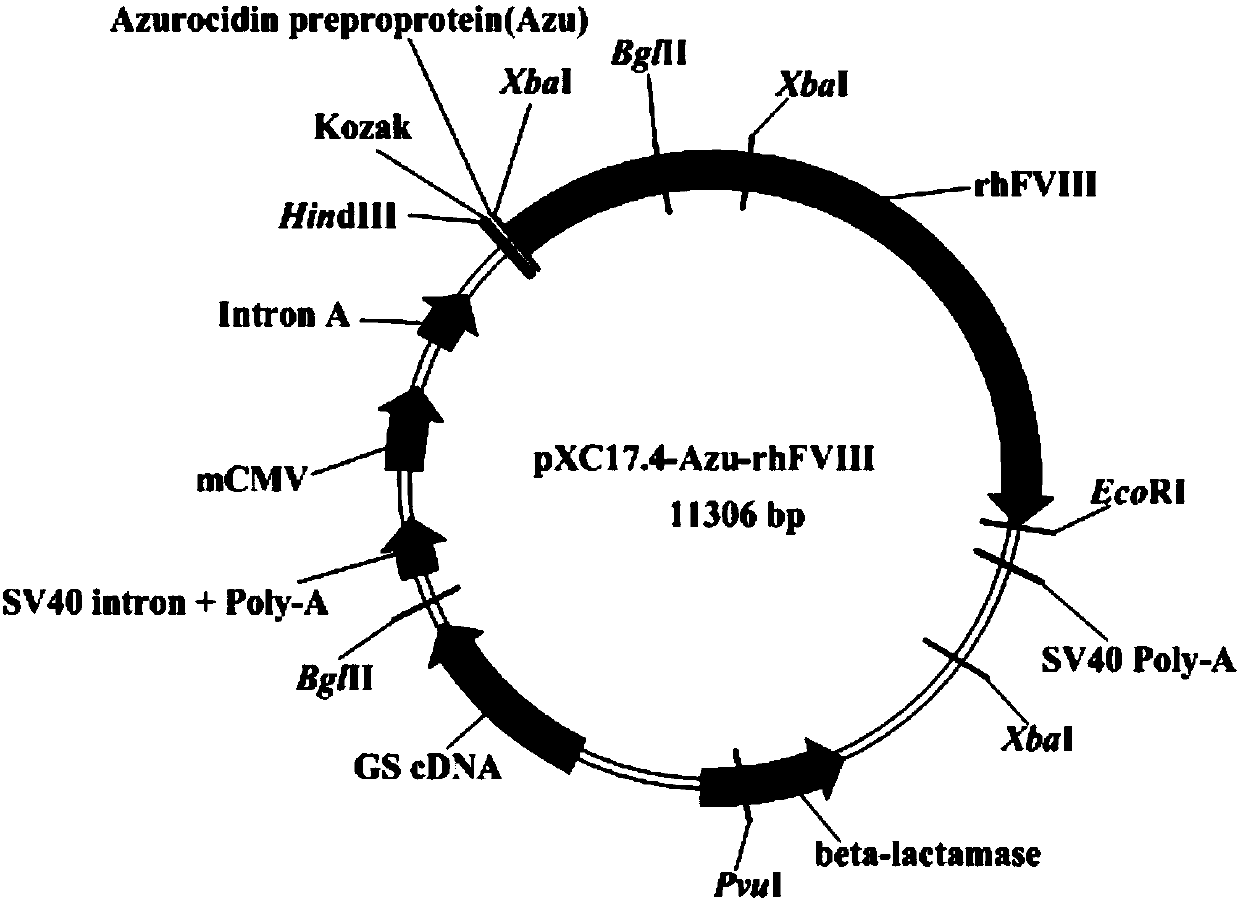
[0035] Example 2 Construction of recombinant expression plasmid pXC17.4-Azu-rhFVIII
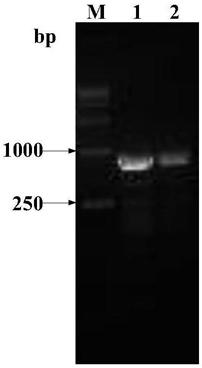
[0036] After the constructed positive plasmid pUC57-Azu-rhFVIII was digested by HindIII and EcoRII, fragment F (SEQ ID NO: 16, 4413bp) containing the target gene Azu-rhFVIII was recovered, and pXC17.4 was digested by HindIII and EcoRII The recovered vector fragment G (SEQ ID NO: 17, 6893 bp) was ligated and screened to obtain the final recombinant expression plasmid pXC17.4-Azu-rhFVIII (11306 bp). The results are shown in figure 1 . The primers were designed at both ends of the final target gene Azu-rhFVIII fragment (4392bp) for sequencing, and the sequencing results showed that the cloned gene fragment was consistent with the theory.
Embodiment 3
[0037] Example 3 Acquisition of positive cells CHOK1SV-KO / pXC17.4-Azu-rhFVIII
[0038] 1. pXC17.4-Azu-rhFVIII electroporation
[0039] The recombinant expression plasmid pXC17.4-Azu-rhFVIII was linearized using the PvuI restriction site, and after purification, filter sterilization and sterility testing, the linearized plasmid pXC17.4-Azu-rhFVIII (concentration: 0.4ug / uL). At the same time, use CD CHO AGT medium (containing 6mM L-Gln) to revive and subculture CHOK1SV-KO cells, and adopt Lonza company GSXceed TM Prepare CHOK1SV-KO recipient cells according to the method in the Gene Expressions system manual, and transfer the linearized plasmid pXC17.4-Azu-rhFVIII into CHOK1SV- KO recipient cells.
[0040] 2. MSX pressurized screening
[0041] After the electric shock is over, transfer the liquid in the electric shock cup to the Erlenmeyer flask of CD CHO AGT medium (without L-Gln), mix gently, place in a shaker, 35-37°C, 140r / min , 8.0%CO 2 to cultivate. After culturin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com