A kind of production method of human antithrombin III
An antithrombin and production method technology, applied in the production field of biological products and blood products, can solve the problems of hidden dangers of product quality, increase of affinity chromatography, shedding of affinity ligands, etc. large amount of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
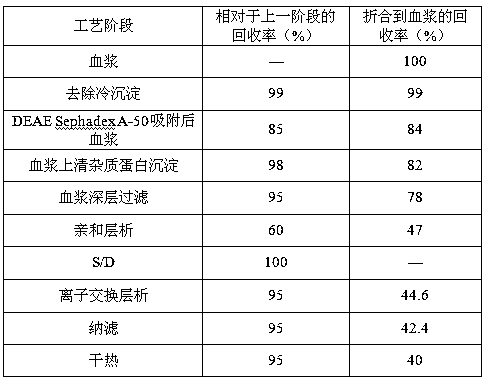
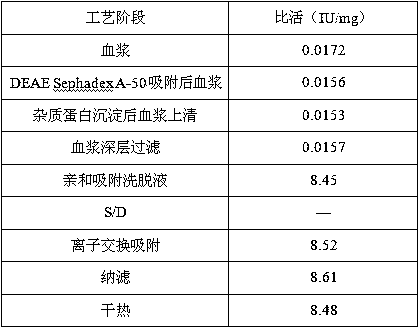
[0035] 1 Fresh frozen human plasma was centrifuged to remove cryoprecipitate, and DEAE Sephadex-A50 gel was adsorbed;
[0036] 1.1 Plasma removal of cryoprecipitate
[0037] Fresh frozen plasma is fed, thawed, and centrifuged to separate cryoprecipitate, and the centrifuged plasma is collected. During the process, the plasma temperature should not exceed 4°C, and the output speed of the centrifuge should not exceed 2L / min.
[0038] 1.2 Adsorption of plasma DEAE Sephadex-A50 gel
[0039] The plasma from which cryoprecipitate was removed was filtered with a 1 μm filter element. Adjust the plasma pH to 7.00; add well-balanced A-50 gel and stir for adsorption for 45 minutes, the adsorption temperature is 4°C; filter out the gel. Equilibrium solution: 0.01mol / L sodium citrate, 0.20mol / L sodium chloride, pH 7.00, 4°C.
[0040] Add 1 times the gel volume of the washing solution, stir and wash twice, filter the washing solution with a 1 μm microporous filter element, and combine i...
Embodiment 2
[0069] plasma depth filtration
[0070] The filter material chooses 3M's 30LA and 60LA combination. Clean the filter material with an aqueous solution containing 5% EDTA at a dosage of 200L / m 2 . Then use 5% sodium citrate solution to clean the filter material, the dosage is 200L / m 2 . Among them, 50L / m is first used 2 Rinse the filter plate with sodium citrate solution, and then use 100L / m 2 Sodium citrate solution to wash out the above solution, and circulate for 30 minutes, and finally use 50L / m 2 Sodium citrate solution to wash out the aforementioned solution. After flushing, drain the solution in the device and perform plasma filtration.
[0071] The filtered plasma was subjected to on-line membrane filtration using a 3M PDA type 0.2 μm double-layer membrane filter element while undergoing column chromatography.
Embodiment 3
[0073] 1 Fresh frozen human plasma was centrifuged to remove cryoprecipitate, and DEAE Sephadex-A50 gel was adsorbed;
[0074] 1.1 Plasma removal of cryoprecipitate
[0075] Fresh frozen plasma is fed, thawed, and centrifuged to separate cryoprecipitate, and the centrifuged plasma is collected. During the process, the plasma temperature should not exceed 4°C, and the output speed of the centrifuge should not exceed 1L / min.
[0076] 1.2 Adsorption of DEAE Sephadex A-50 in plasma
[0077] The plasma from which cryoprecipitate was removed was filtered with a 1 μm filter element. Adjust plasma pH to 7.15; add well-balanced A-50 gel and stir for adsorption for 60 minutes, the adsorption temperature is 2°C; filter out the gel. Equilibrium solution: 0.01mol / L sodium citrate, 0.20mol / L sodium chloride, pH 7.15, 2°C.
[0078] Add 1 times the gel volume of washing solution, stir and wash twice, filter the washing solution with a 1 μm microporous filter element, and combine it with t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com