PEGylated recombinant human granulocyte colony stimulating factor freeze-dried preparation
A technology of colony stimulating factor and PEGylation, applied in the field of biomedicine, can solve the problems of challenging and difficult product quality, uncontrollable particle size, lack of liquid formation process, etc., and achieves short reconstitution time and short production cycle. , the effect of high drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
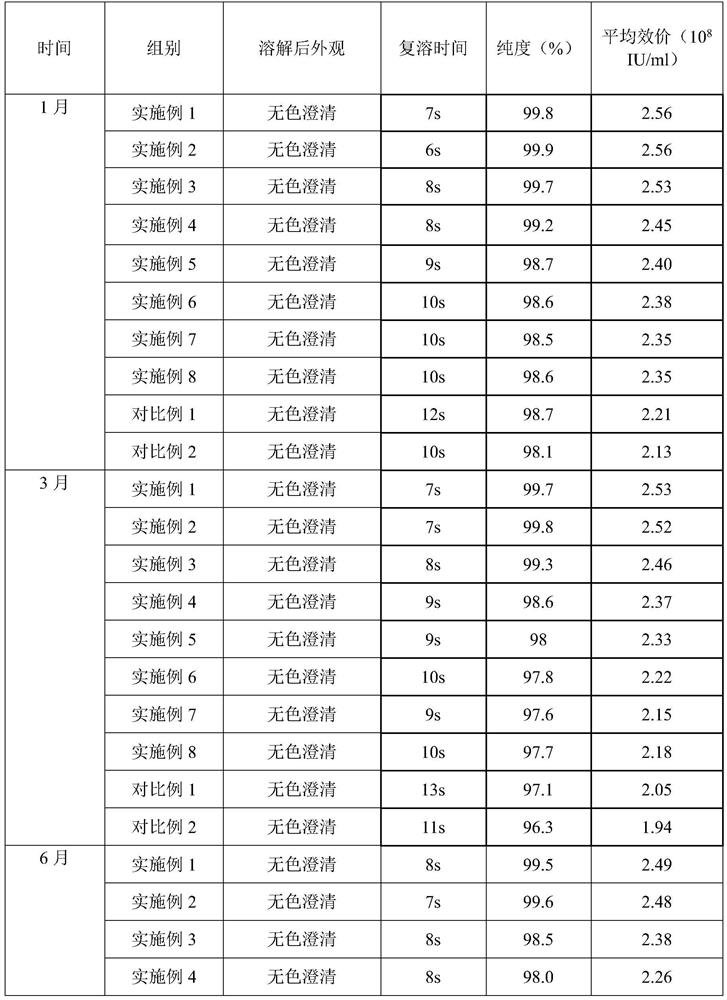
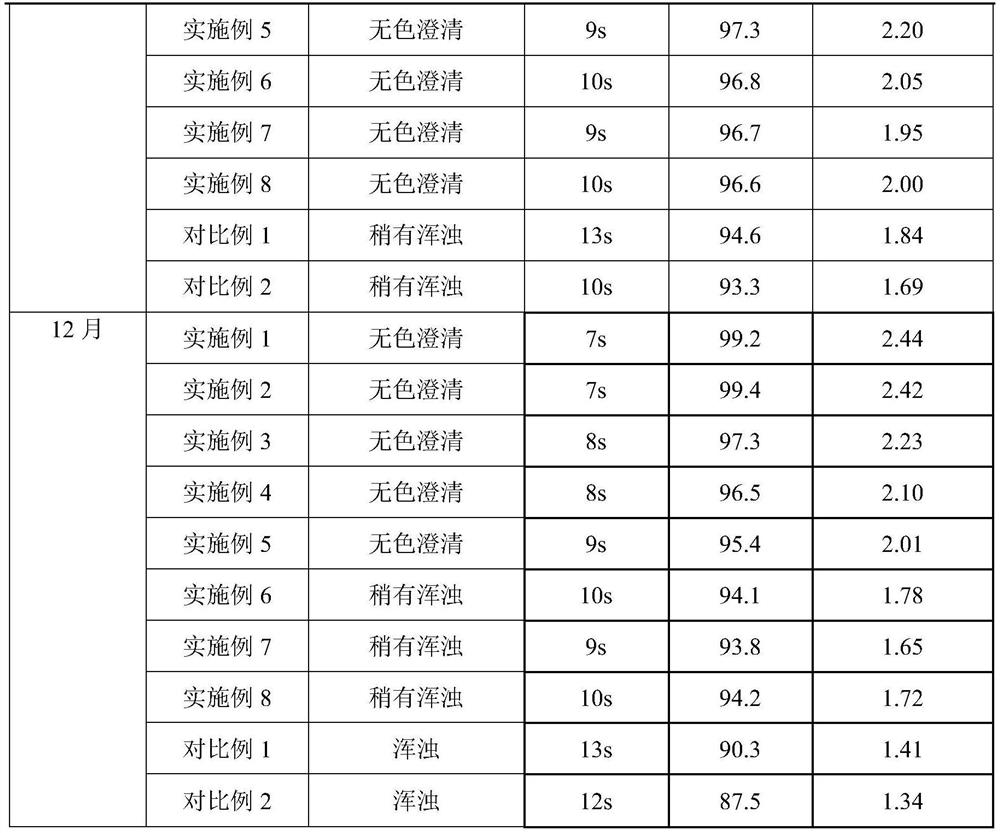
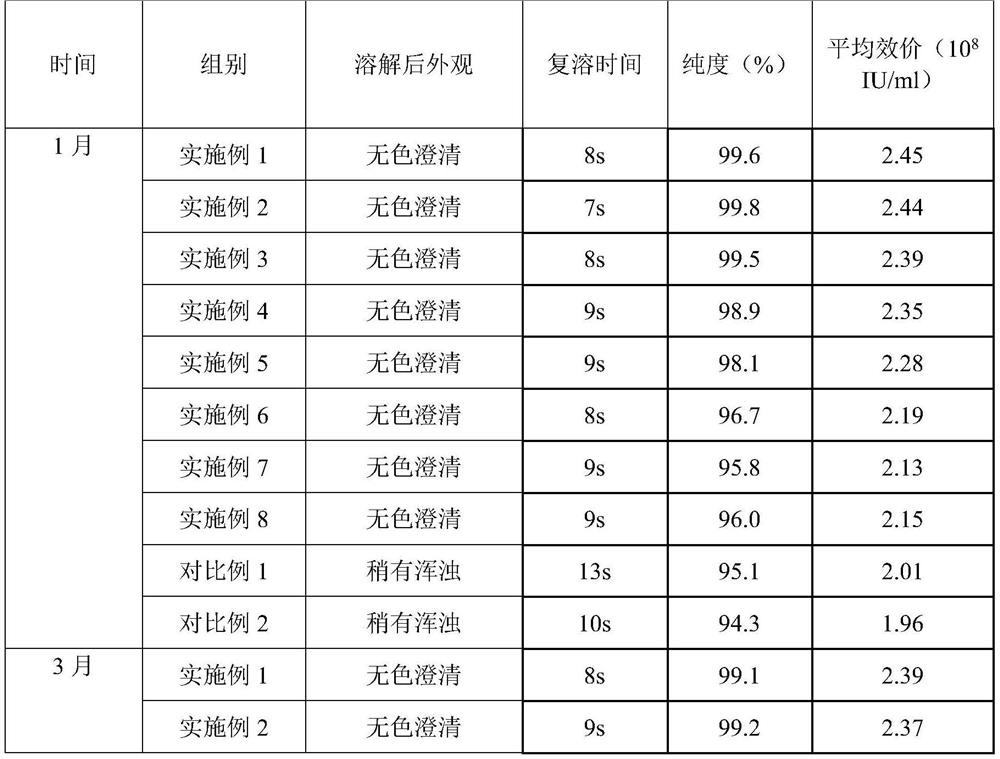
Embodiment 1
[0030] prescription:
[0031] Name Dosage / g
[0032] PEG-rhG-CSF 1
[0033] Sorbitol 3
[0034] Preparation:
[0035] 1) Spray freezing: Dissolve sorbitol and PEG-rhG-CSF in 100ml of water for injection, filter through a 0.22 μm filter membrane, add to a spray bottle, feed flow rate 25mL / min, and conduct spray freezing at -30°C to form Small ice crystals.
[0036] 2) Drying: The pressure in the drying chamber is adjusted to about 20 Pa, the temperature in the drying chamber is -40°C, and the small ice crystals obtained by spray freezing are dried for 8 hours to obtain the finished powder, which is then packaged.
Embodiment 2
[0038] prescription:
[0039] Name Dosage / g
[0040] PEG-rhG-CSF 1
[0041] Sorbitol 2
[0042] Preparation:
[0043] 1) Spray freezing: Dissolve sorbitol and PEG-rhG-CSF in 100ml of water for injection, filter through a 0.22 μm filter membrane, add to a spray bottle, feed at a flow rate of 25mL / min, and conduct spray freezing at -25°C. Small ice crystals are formed.
[0044] 2) Drying: The pressure in the drying chamber is adjusted to about 20 Pa, the temperature in the drying chamber is -30°C, and the small ice crystals obtained by spray freezing are dried for 8 hours to obtain the finished powder, which is then packaged.
Embodiment 3
[0046] prescription:
[0047] Name Dosage / g
[0048] PEG-rhG-CSF 1
[0049] Sorbitol 15
[0050] Preparation:
[0051] 1) Spray freezing: Dissolve sorbitol and PEG-rhG-CSF in 120ml of water for injection, filter through a 0.22 μm filter membrane, add to a spray bottle, feed at a flow rate of 15mL / min, and conduct spray freezing at -35°C. Small ice crystals are formed.
[0052] 2) Drying: The pressure in the drying chamber is adjusted to about 30 Pa, the temperature in the drying chamber is -30° C., and the small ice crystals obtained by spray freezing are dried for 8 hours to obtain the finished powder, which is then packaged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com