Method for preparing human fibrinogen by bilayer chromatography
A human fibrinogen and chromatography technology, which is applied in the field of biopharmaceuticals to achieve the effects of short process flow, high yield and small clinical side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
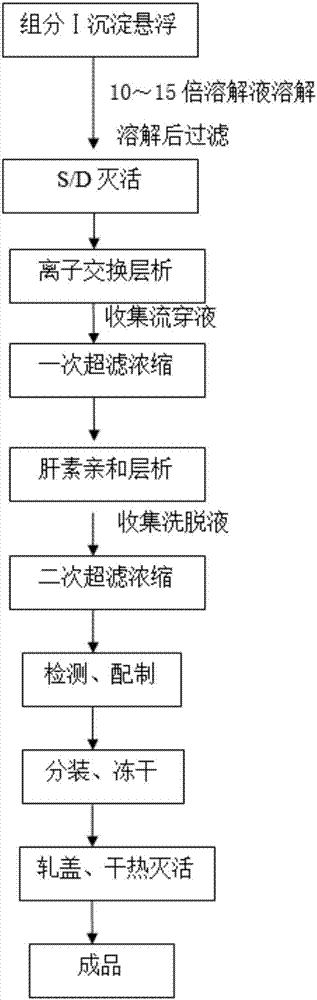
[0025] (1) Precipitation and dissolution of component Ⅰ: Weigh 2.0kg of the precipitate of component Ⅰ, cut it into small pieces with a knife and crush it, add 20kg of dissolving solution, stir in a water bath at 20-26°C for 1.5 hours until completely dissolved, filter with a plate and frame (filter plate The pore size is 0.7μm~1.0μm), and 20.2kg of the filtrate is collected. The solution is: every 1000ml of water for injection contains 12g of sodium citrate, 8.5g of sodium chloride, 4.5g of lysine hydrochloride, 3000IU of heparin sodium, adjusted to pH 6.9 with 1M HCl, and the temperature is 23°C.
[0026] (2) S / D virus inactivation: add S / D solution 2.0L in step (1) filtrate, make polysorbate 80 content be 1.0% in the mixed solution, tributyl phosphate content be 0.3%, in special S Carry out S / D inactivation in the / D inactivation tank, set the temperature at 24.5°C, stir at 100rmp, start timing when the temperature reaches 24.0°C and keep constant, and keep the temperature ...
Embodiment 2
[0035] (1) Precipitation and dissolution of component Ⅰ: Weigh 2.0kg of the precipitate of component Ⅰ, cut it into small pieces with a knife and crush it, add 30kg of dissolving solution, stir in a water bath at 20-26°C for 1.5 hours until completely dissolved, filter with a plate and frame (filter plate The pore size is 0.7μm~1.0μm), and 30.5kg of the filtrate is collected. The solution is as follows: every 1000ml of water for injection contains 12g of sodium citrate, 10g of sodium chloride, 6g of lysine hydrochloride, and 5000IU of heparin sodium. The pH is adjusted to 6.9-7.1 with 1M HCl, and the temperature is 20-26°C.
[0036](2) S / D virus inactivation: add S / D solution 3.05L in step (1) filtrate, make polysorbate 80 content be 1.0% in the mixed solution, tributyl phosphate content be 0.3%, in special S Carry out S / D inactivation in the / D inactivation tank, set the temperature at 24.5°C, stir at 100rmp, start timing when the temperature reaches 24.0°C and keep constant,...
Embodiment 3
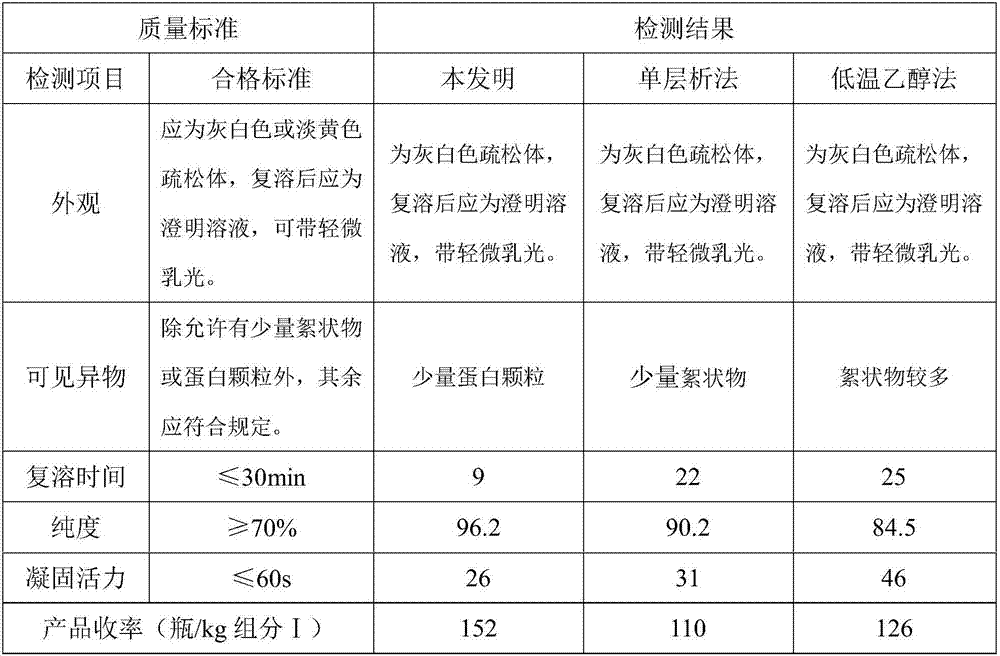
[0044] Embodiment 3: comparative example, traditional low temperature ethanol method, concrete preparation process is as follows:
[0045] (1) Primary suspension and filtration of component I: Weigh 2.0 kg of component I precipitate, cut into small pieces with a knife and crush, add 20 kg of primary suspension, stir in a water bath at 24°C to 26°C for 1.5 hours until completely dissolved, and plate Filtration (filter plate pore size 0.7 μm ~ 1.0 μm). The primary suspension is: 1000ml of water for injection contains 12g of sodium citrate, 8.5g of sodium chloride, 2.7g of Tris, 11g of sucrose, 4.4g of lysine hydrochloride, and the pH is adjusted to 6.7-7.0 with 1M HCl.
[0046] (2) S / D virus inactivation: add S / D solution by 1 / 10 of step (1) gained filtrate weight, make polysorbate 80 content be 1.0% in the mixed solution, tributyl phosphate content be 0.3%, Carry out S / D inactivation in a special S / D inactivation tank, set the temperature at 24.5°C, stir at 100rmp, start timin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com