Cetrorelix pharmaceutical composition and preparation method thereof
A technology of cetrelix and cetrelix acetate, applied in the field of cetrerelix pharmaceutical composition and preparation thereof, can solve problems such as increase of related substances, poor stability, etc., achieve high stability, improve safety, shorten the time The effect of reconstitution time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0014] An embodiment of the present invention provides a method for preparing a cetrorelix pharmaceutical composition, the preparation method comprising: dissolving cetrorelix acetate with hydrochloric acid to obtain a cetrorelix acetate hydrochloride solution;
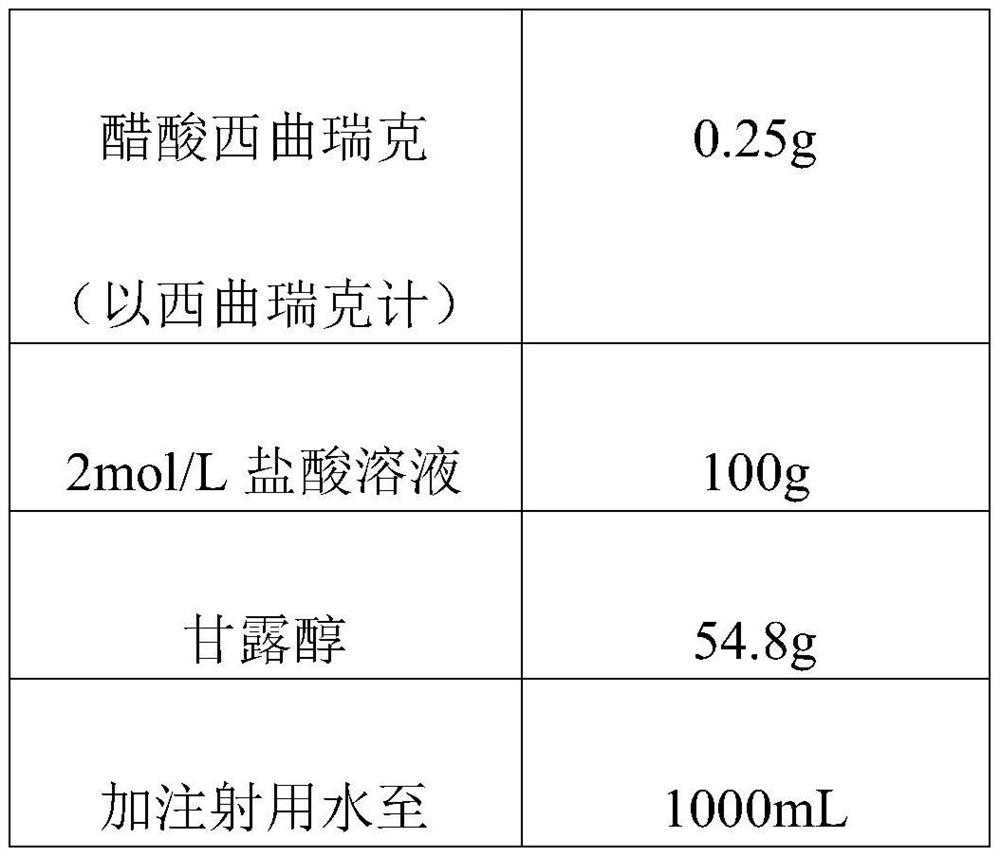
[0015] In the cetrorelix acetate hydrochloric acid solution, the final concentration of the hydrochloric acid is 0.05-3 mol / L.
[0016] Specifically, cetrorelix acetate is a compound, the English name is Cetrorelix acetate, and the molecular formula is C 70 h 92 CLN 17 o 142 (C 2 h 4 o 2 ), the molecular weight is 1551.16Da, CAS NO.130143-01-0. available through existing channels. Cetrorelix acetate for injection is indicated for patients undergoing controlled ovarian stimulation to prevent premature ovulation, and then to perform egg collection and assisted reproductive technology treatment.
[0017] Hydrochloric acid, the aqueous solution of hydrogen chloride (HCl), belongs to the monobasic inorganic strong ...
Embodiment 1
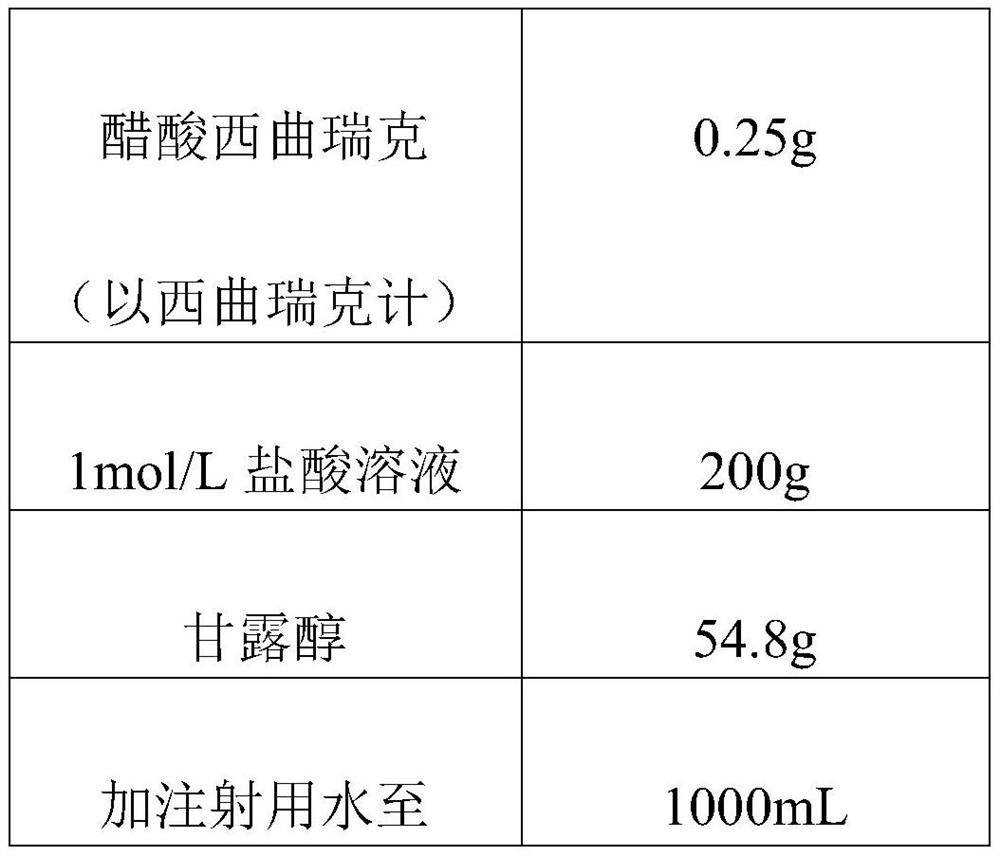
[0035] The present invention provides a preparation method of a cetrorelix pharmaceutical composition, whose components refer to Table 1.
[0036] Table 1 Drug Components
[0037]
[0038] The preparation method of the cetrorelix pharmaceutical composition is as follows:
[0039] According to the weight or volume shown in Table 1, obtain the raw material components;
[0040] After dissolving cetrorelix acetate with 1mol / L hydrochloric acid solution, add 600mL water for injection and mix well; then add mannitol to dissolve and mix well; add water for injection to a total volume of 1000mL to obtain grams of mixed solution.
[0041] The prepared mixed solution containing mannitol and cetrorelix acetate was sub-packaged and filtered, with 1.0 mL / bottle for sub-packaging, and a 0.2 μm filter was used for filtration.
[0042]After filtering, the plate layer temperature was lowered to -40°C, and then freeze-dried to obtain a freeze-dried pharmaceutical composition.
Embodiment 2
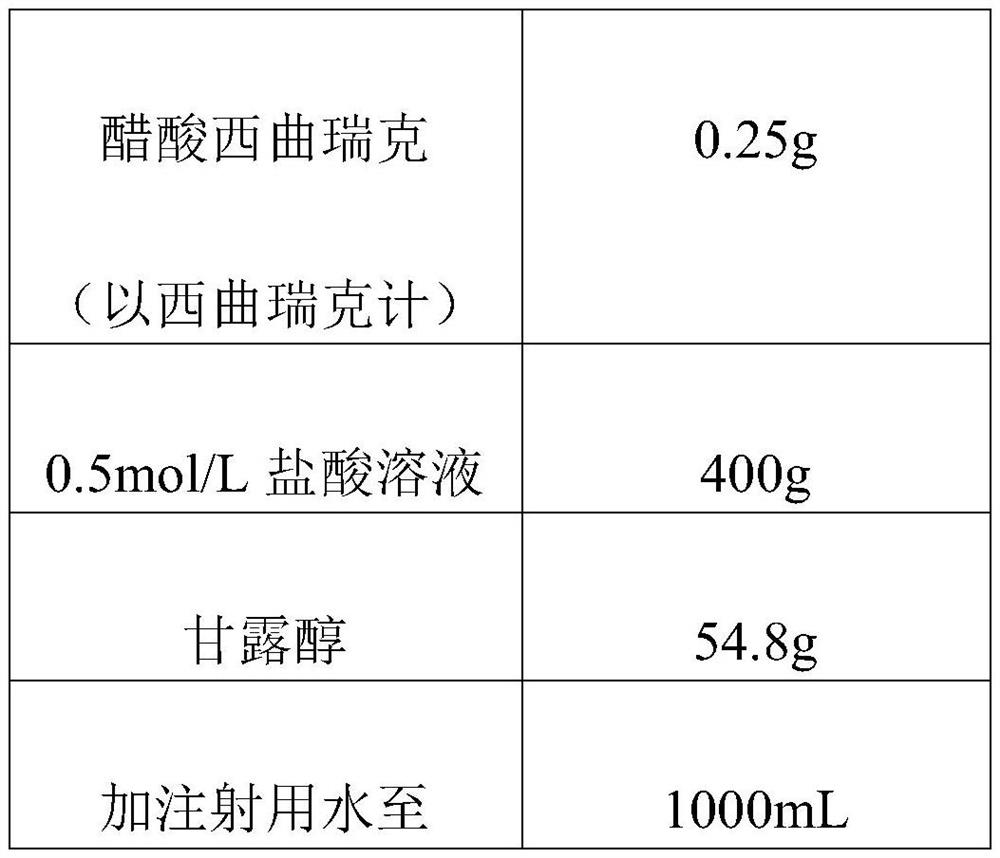
[0044] This embodiment provides a preparation method of a cetrorelix pharmaceutical composition, which is roughly the same as that of Example 1, except that the proportion of the components is different, and the difference is as follows:
[0045] Table 2 Drug Components
[0046]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com