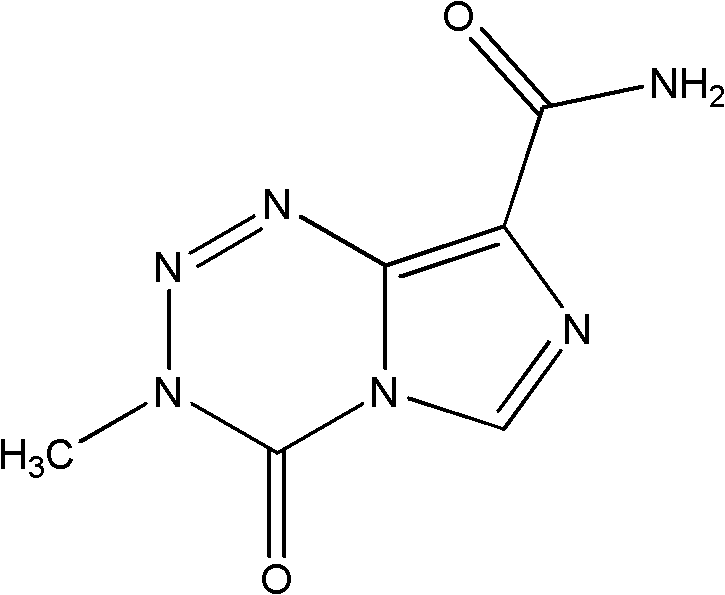
Lyophilized preparation of temozolomide and its preparation method
A technology for temozolomide and preparations, which is applied in the field of pharmaceutical preparations and can solve problems such as long reconstitution time and insufficient stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
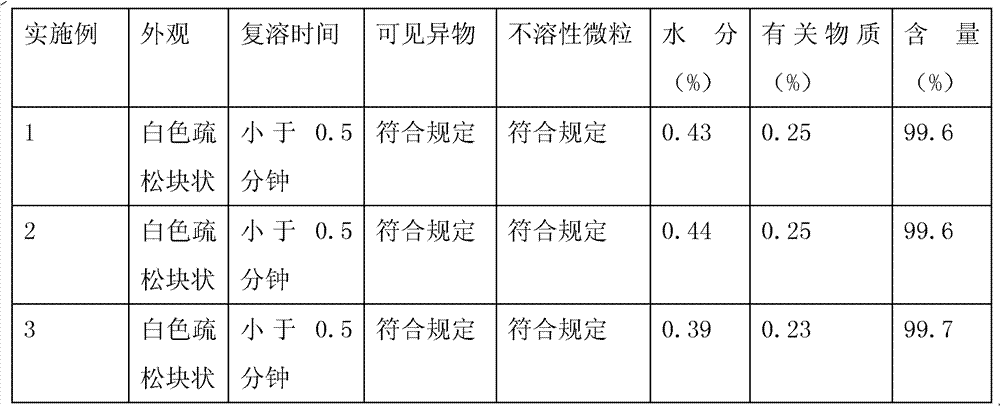
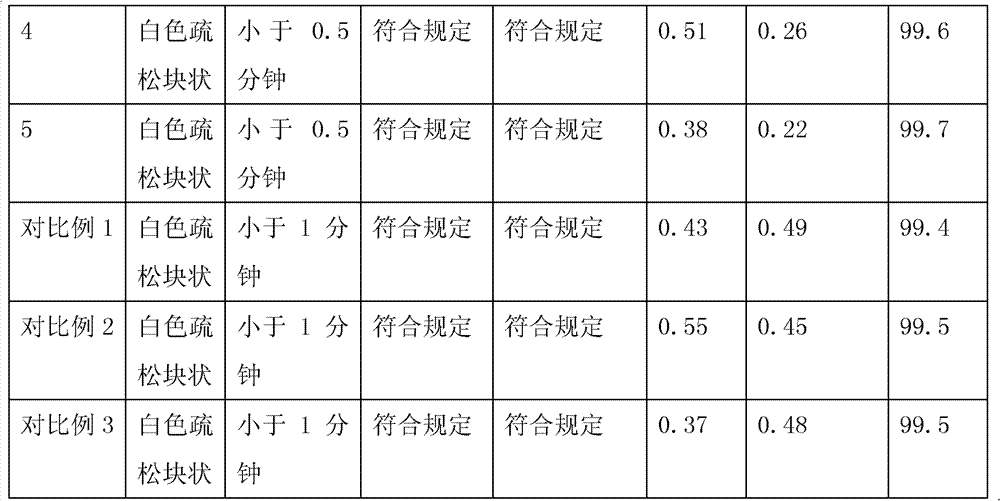
Embodiment 1
[0028] Preparation composition:
[0029] sodium citrate
[0030] Preparation method: Measure 4000ml of water for injection, add 60g of mannitol, 32g of nicotinamide, 12g of polysorbate-80, 24g of sodium citrate, 20g of hydrochloric acid, stir and mix evenly, add 10g of temozolomide, stir to completely dissolve temozolomide, and the solution Sterilize and filter through a 0.22μm microporous membrane, divide into 100ml vials, fill each bottle with 40ml, partially plug with butyl rubber stoppers, and place in a plate; place in a freeze dryer, and reduce the sample temperature to -45°C And keep it for 3 hours, turn on the vacuum system, when the vacuum degree of the front box reaches below 20Pa, start freeze-drying, and raise the temperature of the shelf to -20°C within 1 hour and maintain it for 15 hours, and then make the shelf dry within 1 hour Raise the temperature to -2°C and maintain it for 25 hours, raise the shelf temperature to 30°C within 1 hour and ma...
Embodiment 2
[0032] Preparation composition:
[0033] Preparation method: with embodiment 1
Embodiment 3
[0035] Preparation composition:
[0036] Temozolomide
[0037] Preparation method: with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com