Preparation method of betamethasone sodium phosphate freeze-dried powder injection
A technology of sodium metasone phosphate and freeze-dried powder injection, which is applied in freeze-dried delivery, medical preparations containing active ingredients, powder delivery, etc., can solve problems such as low production efficiency and high impurities, and achieve improved production efficiency and stability. Good, shorten the effect of production time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
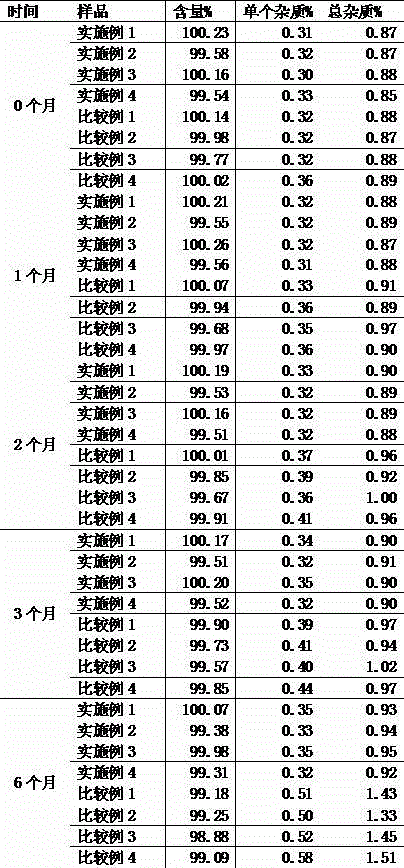
Embodiment 1
[0032] A preparation method of betamethasone sodium phosphate freeze-dried powder injection, comprising:
[0033] Prepare materials :
[0034] Betamethasone Sodium Phosphate 2.63g
[0035] Mannitol 20g
[0036] Proper amount of dilute hydrochloric acid or sodium hydroxide solution
[0037] Water for injection to 1000ml,
[0038] preparation :
[0039] (1) Add 800ml of water for injection to dissolve mannitol,
[0040] (2) Add betamethasone sodium phosphate to the solution of the above step (1) to dissolve, add water for injection to 1000ml, adjust the pH value to 7.0~9.0 with dilute hydrochloric acid or sodium hydroxide solution,
[0041] (3) Filter the medicinal liquid obtained in the above steps with a 0.45 μm microporous membrane, then fine filter with a 0.22 μm microporous membrane, and then fill it into a control bottle, each 1ml, half stoppered, and enter the next step of freezing dry,
[0042] (4) Freeze-drying is performed as follows:
[0043] (4.1) Pre-fre...
Embodiment 2
[0048] A preparation method of betamethasone sodium phosphate freeze-dried powder injection, comprising:
[0049] Prepare materials :
[0050] Betamethasone Sodium Phosphate 5.26g
[0051] Mannitol 40g
[0052] Proper amount of dilute hydrochloric acid or sodium hydroxide solution
[0053] Water for injection to 1000ml,
[0054] preparation :
[0055] (1) Add 800ml of water for injection to dissolve mannitol,
[0056] (2) Add betamethasone sodium phosphate to the solution of the above step (1) to dissolve, add water for injection to 1000ml, adjust the pH value to 7.0~9.0 with dilute hydrochloric acid or sodium hydroxide solution,
[0057] (3) Filter the medicinal liquid obtained in the above steps with a 0.45 μm microporous membrane, then fine filter with a 0.22 μm microporous membrane, and then fill it into a control bottle, each 1ml, half stoppered, and enter the next step of freezing dry,
[0058] (4) Freeze-drying is performed as follows:
[0059] (4.1) Pre-freez...
Embodiment 3
[0064] A preparation method of betamethasone sodium phosphate freeze-dried powder injection, comprising:
[0065] Prepare materials :
[0066] Betamethasone Sodium Phosphate 5.26g
[0067] Lactose 20g
[0068] Proper amount of dilute hydrochloric acid or sodium hydroxide solution
[0069] Water for injection to 1000ml,
[0070] preparation :
[0071] (1) Add 800ml of water for injection to dissolve lactose,
[0072] (2) Add betamethasone sodium phosphate to the solution of the above step (1) to dissolve, add water for injection to 1000ml, adjust the pH value to 7.0~9.0 with dilute hydrochloric acid or sodium hydroxide solution,
[0073] (3) Filter the medicinal liquid obtained in the above steps with a 0.45 μm microporous membrane, then fine filter with a 0.22 μm microporous membrane, and then fill it into a control bottle, each 1ml, half stoppered, and enter the next step of freezing dry,
[0074] (4) Freeze-drying is performed as follows:
[0075] (4.1) Pre-freez...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com