Preparation method of voriconazole for injection
A technology for voriconazole and injection, which is applied in the field of preparation of voriconazole for injection, which can solve the problems of uneven finished product, long freeze-drying cycle, rough powder cake, etc., and achieve the effects of improving clarity, improving crystal form, and promoting uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
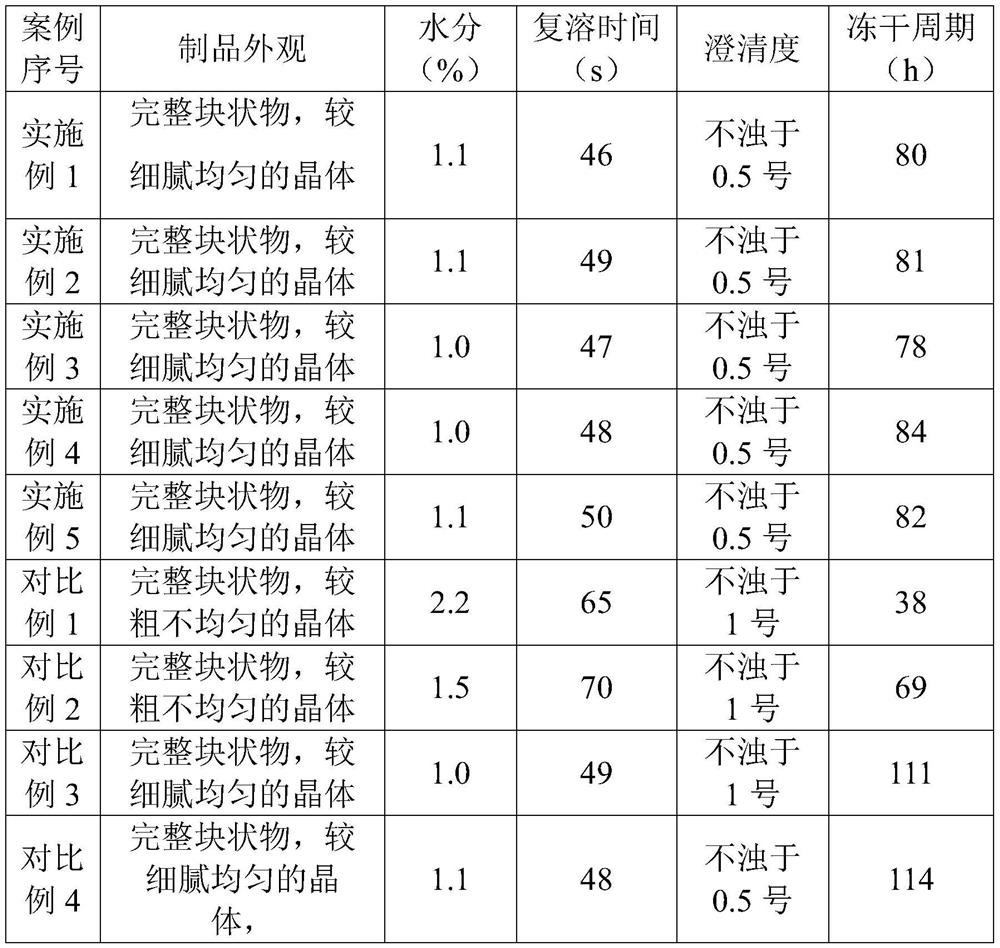
Image
Examples
Embodiment 1
[0019] 1) Preparation: Weigh voriconazole and sulfobutylbetacyclodextrin (SBECD) at a weight ratio of 1:18, add SBECD to an appropriate amount of water, add voriconazole after SBECD is completely dissolved, and continue stirring until completely dissolved and mixed evenly. Obtain a sample containing voriconazole;
[0020] 2) Sterile filtration, half-stoppering for filling: filter the sample to the aseptic filling room with 0.45 μm and 0.22 μm sterile filters, fill in 25ml vials, and half-stopper;
[0021] 3) vacuum freeze drying:
[0022] a. Overcooling: place the subpackaged samples on the partition of the freeze dryer, set the temperature of the partition to 0°C, and keep at this temperature for 4 hours;
[0023] b. Pre-freezing: set the partition temperature to -45°C and keep at this temperature for 3 hours;
[0024] c. Annealing treatment: Raise the temperature of the separator to -10°C, keep it at this temperature for 5h, and then lower it to -45°C and keep it for 4h; ...
Embodiment 2
[0030] 1) Preparation: Weigh voriconazole and SBECD according to a weight ratio of 1:15, add SBECD into water, add voriconazole after SBECD is completely dissolved, and continue stirring until completely dissolved and mixed uniformly to obtain a voriconazole-containing sample;
[0031] 2) Sterile filtration, half-stoppering for filling: filter the sample to the aseptic filling room with 0.45 μm and 0.22 μm sterile filters, fill in 25ml vials, and half-stopper;
[0032] 3) vacuum freeze drying:
[0033] a. Overcooling: place the subpackaged samples on the partition of the freeze dryer, set the temperature of the partition to -5°C, and keep at this temperature for 2 hours;
[0034] b. Pre-freezing: set the partition temperature to -42°C and keep at this temperature for 4 hours;
[0035] c. Annealing treatment: Raise the temperature of the separator to -5°C, keep it at this temperature for 3 hours, and then lower it to -40°C and keep it for 6 hours;
[0036] d. Vacuuming: Turn ...
Embodiment 3
[0041] 1) Preparation: Weigh voriconazole and SBECD according to a weight ratio of 1:16, add SBECD to an appropriate amount of water, add voriconazole after SBECD is completely dissolved, and continue stirring until completely dissolved and mixed evenly to obtain a voriconazole-containing sample;
[0042] 2) Sterile filtration, half-stoppering for filling: filter the sample to the aseptic filling room with 0.45 μm and 0.22 μm sterile filters, fill in 25ml vials, and half-stopper;
[0043] 3) vacuum freeze drying:
[0044] a. Overcooling: place the subpackaged samples on the partition of the freeze dryer, set the temperature of the partition to -4°C, and keep at this temperature for 3 hours;
[0045] b. Pre-freezing: set the partition temperature to -40°C, and keep at this temperature for 6 hours;
[0046] c. Annealing treatment: Raise the temperature of the separator to -8°C, keep it at this temperature for 4 hours, and then lower it to -43°C and keep it for 5 hours;
[0047...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com